Nickel, Ni, atomic number 28
Nickel price, occurrence, extraction and use
Nickel is a chemical element with the element symbol Ni and the atomic number 28. It belongs to the transition metals, in the periodic table it stands after the older counting method in the 8. Subgroup or iron-platinum group, according to the newer in the group 10 or nickel group.
History
Nickel was first represented purely by Axel Frederic Cronstedt 1751 and named after the mineral cupronickel (Swedish kopparnickel, today Nickelin) in which he found the hitherto unknown metal.
The medieval miners used the term cupronickel to refer to the ore, which looked like copper ore, but from which no copper could be obtained, as if bewitched by mountain spirits ("nickel"). A similar kobold-like etymology can be found in Cobalt.
The first coin of pure nickel was coined 1881.
occurrence
Nickel occurs in the earth's crust with a content of about 0,008%. Geophysical and geochemical evidence suggests that most of the nickel is located on Earth and other terrestrial planets in the core, where it forms an alloy with iron and some light elements. In the Earth's core, its mass fraction according to the latest models is about 5,2%.
Solid, that is in elemental form nickel is rare. So far, only about 50 locations for solid nickel documented (2018), including in Australia, China, Canada, Russia and the United States of America.
Traditionally, the majority of nickel production is from sulphidic ores such as pentlandite (about 34% nickel), nickel magnetic gravel (pyrrhotite and pentandite agglomerate) and some other nickel minerals such as millerite (about 64-65% nickel) and nickelin (about 44% Nickel) won. In addition, lateritic nickel ores, mainly from Garnierit, a mixture of Népouit (about 46% nickel) and Willemseit (about 29% nickel), are mined as raw materials for nickel production. Overall, some 200 nickel minerals are known to date, and some have much higher nickel contents than those already mentioned, but are much less common than these. For example, the very rare bunsenite is the mineral with the highest nickel content of up to 78,58%. The equally rare Minerals Heazlewoodite and Awaruit contain between 72 and 73% nickel.
The extraction is shifting due to the exploitation of the classic sulfidic deposits increasingly to lateritic nickel ores. However, these must be laboriously obtained by high pressure acid leaching (English high pressure acid leaching).
To be able to degrade the nickel economically, the nickel content of the ore must be at least 0,5%. The most important occurrences are found in Canada (Sudbury Basin), New Caledonia, Russia (Norilsk and Kola Peninsula), Australia (Queensland) and Cuba (Moa Bay and Nicaro). A common companion to nickel is cobalt.
Nickel as a mineral

Naturally occurring nickel in its elemental form was first described by Paul Ramdohr 1967 and recognized by the International Mineralogical Association (IMA) as an independent mineral species (internal input number of the IMA: 1966-039).
According to the classification of minerals according to Strunz (9 edition) nickel under the system no. 1.AA.05 (Elements - Metals and Intermetallic Compounds - Copper Cupalite Family - Copper Group) respectively in the obsolete 8. Edition listed under I / A.04b (nickel series). The classification of minerals according to Dana, which is predominantly used in English-speaking countries, leads the element mineral under the system no. 01.01.11.05 (iron-nickel group).
The type of locality is the Bogota peninsula near Canala in the northern province of New Caledonia, where native nickel in the form of idiomorphic cubic grains or ingrown cubes to about 0,1 mm as inclusions in Heazlewoodite and as a "spidery" irregular mass between the Heazlewooditkörnern found. Accompanying minerals in addition to Heazlewoodite include chalcopyrite, chalcocite, galena, Godlevskit, solid copper, millerite, orcelite, pentlandite, pyrite and pyrrhotite occur.
Extraction and presentation
The majority of nickel is derived from nickel and copper-bearing iron ores such as nickel magnetic gravel. In order to make the extraction economically, the nickel must first be enriched by flotation to about five percent nickel content. Then the ore is roasted similar to the copper production. Here, the ore is first pre-roasted to convert a portion of the iron sulfide into iron oxide. Subsequently, silicates and coke are added to slag the iron oxide as iron silicate. At the same time, the copper-nickel raw stone is formed from nickel, copper and iron sulphide. Since this is specifically heavier than the iron silicate slag, the two phases can be tapped separately.
Subsequently, the rough stone is filled in a converter and silica is added. It is injected with oxygen. As a result, the remaining iron sulfide is roasted to iron oxide and then scrubbed. The result is the copper-nickel fine stone, which consists of about 80% of copper and nickel and about 20% of sulfur.
| Rank | Country | Production (in million t) |
|---|---|---|
| 1 | Indonesia | 400.000 |
| 2 | Philippines | 230.000 |
| 3 | Caledonia | 210.000 |
| 4 | Canada | 210.000 |
| 5 | Australia | 190.000 |
| 6 | Russia | 180.000 |
| 7 | Brazil | 140.000 |
| 8 | People's Republic of China | 98.000 |
| 9 | Guatemala | 68.000 |
| 10 | Cuba | 51.000 |
Extraction of Rohnickel
To recover the Rohnickels the nickel must be separated from the copper. For this purpose, the fine stone is fused with sodium sulfide Na2S. Only a slight melting double sulfide forms between copper and sodium sulfide. Two easy-to-separate phases of copper-sodium double sulfide (liquid) and nickel sulfide are formed. After separation, the nickel sulfide is roasted to nickel oxide and then reduced to nickel with coke.
To recover pure nickel, the Rohnickel is refined by electrolysis. For this purpose, the Rohnickel is connected as an anode, a Nickelelfeinblech as a cathode in an electrolytic cell. The electrolyte used is a nickel salt solution. During electrolysis, nickel and all the less noble components go into solution at the anode. All nobler components remain solid and fall under the electrode as anode sludge. This serves as an important source for the production of precious metals, such as gold or platinum. At the cathode, nickel ions from the solution are reduced to nickel, all the less noble components remain in solution. The purity of electrolytic nickel is around 99,9%.
For the extraction of pure nickel with a purity of 99,99% there is a special process the moon process, named after Ludwig Moon, who discovered 1890 nickel tetracarbonyl. This process is based on the formation and decomposition of nickel tetracarbonyl. For this purpose, finely divided Rohnickel powder is brought into a carbon monoxide stream at 80 ° C. This forms gaseous nickel tetracarbonyl. This is freed from flue dust and sent to an 180 ° C hot decomposition chamber. Inside are small nickel balls. At this point, the nickel tetracarbonyl decomposes again to nickel and carbon monoxide. This results in very pure nickel.
Labor representation
There are different methods for representing small amounts of very pure nickel in the laboratory:
Reduction of the oxide with hydrogen at 150 ° C to 250 ° C:
Reduction of a nickel (II) chloride suspension in diethyl ether via a Grignard reaction
Thermal decomposition of nickel (II) oxalate in the absence of oxygen:
Reduction of nickel (II) chloride with a sodium dispersion:
In particular, the thermolysis of the oxalate provides finely divided pyrophoric nickel powder.
Features
Physical Properties
Nickel is a silvery-white metal that is one of the heavy metals with a density of 8,91 g / cm3. It is medium hard (Mohs hardness 3,8), forgeable, ductile and can be polished very well. Nickel, like iron and cobalt, is ferromagnetic, with a Curie temperature of 354 ° C. The metal crystallizes in a cubic-face-centered crystal structure (copper type) in the space group Fm3m (space group number 225) with the lattice parameter a = 352,4 pm and four formula units per unit cell. This structure retains it even at high pressures up to at least 70 GPa. Another metastable modification with cubic-body-centered spherical packing could be obtained in thin layers on iron or gallium arsenide. It has a significantly lower Curie temperature with 183 ° C.
The tensile strength of annealed nickel in 400-450 MPa is at an elongation at break between 30 and 45%. The hardness values are around the 80 HB. Cold-strengthened nickel whose elongation at break is below 2% achieves strengths up to 750 MPa with hardness values around 180 HB. Pure nickel semi-finished products with 99% Ni content can be cold strengthened.
The isotope 62Ni has the highest binding energy per nucleon of all isotopes of all elements.
Chemical properties
Nickel is very stable at room temperature to air, water, hydrochloric acid and alkalis. Diluted acids attack nickel only very slowly. Compared with concentrated, oxidizing acids (nitric acid), passivation occurs analogously to stainless steel. Nickel is soluble in dilute nitric acid (about 10 to 15 percent). Even a half-concentrated nitric acid (about 30 percent) causes noticeable passivation. The most common oxidation state is + II, more rarely -I, 0, + I, + III and + IV are observed. In nickel tetracarbonyl, nickel has the oxidation number 0. Nickel (II) salts dissolve in water to form aqua complexes of greenish color.
Finely divided nickel reacts with carbon monoxide at 50 to 80 ° C to form nickel tetracarbonyl, Ni (CO) 4, a colorless, very toxic liquid. This serves as an intermediate for the production of pure nickel by the moon process. At 180 to 200 ° C, nickel tetracarbonyl decomposes back into nickel and carbon monoxide.
physiology
The controversial essentiality of nickel is contrasted with the existence of several enzymes that normally contain nickel, but are not dependent on it because its role as a cation can be taken over by other divalent cations. In humans, these are three proteins known to bind nickel:
alpha-fetoprotein binds nickel, but does not depend on it, as there is no enzyme
Acireductone dioxygenase, an enzyme of the methionine salvage pathway that typically binds nickel or another divalent cation
Polyribonucleotide 5'-hydroxyl kinase Clp1, which requires as cofactor magnesium, manganese or nickel
For plants and various microorganisms, the essentiality of nickel is due to the isolation of several enzymes (eg urease, Co-F430) containing nickel in the active site, as well as by detection of deficiencies in low-nickel environments, which are enhanced by the addition of Ni (II ) Salts, secured.
In electrophysiology, nickel ions are used to block voltage-activated calcium channels.
Health problems
Nickel is the most common cause of contact allergy with nickel dermatitis: in Germany, an estimated 1,9 to 4,5 million people are sensitized to nickel. Because of this, metals and alloys that come into contact with the skin are increasingly less nickel-plated. About 10% of all children are sensitized to nickel. Upon renewed contact with the allergen, they may react with a contact allergy.
According to the European Food Safety Authority (EFSA), the tolerable daily intake (TDI) of nickel is 2,8 micrograms (0.0028 milligrams) per kilo of body weight. 2019 has had the Upper Austrian Chamber of Labor examine twelve different soy drinks at the Agency for Health and Food Safety. The values were between 0,25 (Dennree soy drink nature) and 0,69 milligrams per liter (yes, of course organic soy drink). In the highest-calorie soymilk, a 30 kilo of heavy children has already consumed more than twice as much nickel in quarter-liter as recommended by EFSA.
Inhalation of inorganic nickel compounds is associated with an increased risk of cancer for squamous cell carcinoma of the lung and upper respiratory tract. Such malignant neoplasms are recognized as occupational diseases in Germany on occupational exposure (BK 4109). In addition, increased nickel content in the air and drinking water is a risk factor for nickel sensitization in children.
The use of nickel in consumer goods (such as wristwatches, toys, food processing equipment, etc.) is limited by regulation in the European Union. This has been implemented in Germany by the Consumer Goods Ordinance, which sets limit values for release.
Usage
Nickel is needed as metal in small quantities, most of the production goes into the production of stainless steels and nickel alloys. Nickel is used in many specific and identifiable industrial and consumer goods, including stainless steel, Alnico magnets, coins, rechargeable batteries, electric guitar strings, microphone capsules, plating on plumbing fixtures, and special alloys such as Permalloy, Elinvar, and Invar. It is used for coating and as a tint in glass. The reserves of nickel deposits depleted from today's point of view lie between 70 and 170 million tons. Currently, more than one million tonnes (2006: 1,340 million tonnes) are being produced worldwide each year. The price of nickel is at times subject to very high price fluctuations due to financial market speculation.
Around 25 percent of the world nickel deposit is in New Caledonia, a French overseas territory.

Nickel cadmium battery, picture Wikipedia
Use as metal
Pure nickel metal is used in finely divided form as a catalyst in the hydrogenation of unsaturated fatty acids. Due to its chemical resistance, nickel is used for apparatus in the chemical laboratory and the chemical industry (eg nickel crucibles for digestions). From nickel metal nickel alloys, z. For coins.

Nickel wire (wire), nickel wire can be pulled up to 0,01mm thin
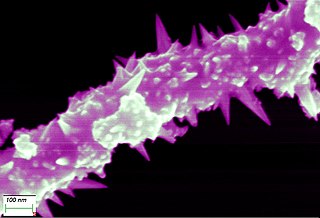
Nickel wire 0,01mm in the consideration by a high performance microscope
Nickel serves as a coating metal for the corrosion protection ("nickel plating") of metal objects: Because of its oxidation-protective properties, metals (in particular iron) are coated with a nickel layer for certain technical purposes by means of galvanic technology.
The metal was also used earlier to make the frames of nickel goggles.
As a beta emitter, the nickel isotope 63Ni is used in electron capture detectors in gas chromatographs.
Use as alloy
Nickel is an important alloying metal that is mainly used for steel finishing. Most of the nickel goes there. It makes steel resistant to corrosion and increases its hardness, toughness and ductility. Steels high-alloyed with nickel are used in particularly corrosive environments. The stainless steel V2A (the name comes from the "test batch 2 austenitic" in the Krupp steelworks, corresponds to X12CrNi18-8) contains 8% nickel in addition to 18% chromium, V4A (brand names Cromargan or Nirosta) 11% in addition to 18% chromium and 2% molybdenum.

Nickel coins picture Wikipedia
Nickel is an excellent alloying agent for certain precious metals and is used in the fire test as a collector of platinum group elements. As such, nickel is capable of fully collecting all six platinum group elements, especially platinum and palladium, from ores and partially collecting gold.
Nickel foam or nickel mesh is used in gas diffusion electrodes for alkaline fuel cells.
Nickel and its alloys are often used as catalysts for hydrogenation reactions. Raney nickel, a finely divided nickel-aluminum alloy, is a common form, although related catalysts are also used, including Raney type catalysts.
About 20% of the nickel is used (in Germany) for the production of other nickel alloys:
Konstantan, an alloy of 55% copper and 45% nickel, which has an approximately constant electrical resistivity over a wide temperature range. It is mainly used for accurate resistances.
Nickel-base superalloys are alloys especially for use at high temperatures and under corrosive media. They are used, for example, in aircraft turbines and gas turbines of power plants.
Raney nickel, a nickel-aluminum alloy that is an important catalyst for the hydrogenation of organic compounds.
Nickel silver, a copper-nickel-zinc alloy with 10-26% nickel content, which is particularly resistant to corrosion and is mainly used for cutlery and electrotechnical equipment.
Monel, also a copper-nickel alloy with about 65% nickel, 33% copper and 2% iron, which is characterized by particular chemical resistance, including fluorine. It is therefore used for fluorine gas cylinders.
Austenitic ductile iron, a special spheroidal cast iron with up to 20% nickel, for use in corrosive environments and at high temperatures.
proof
The detection reaction for the nickel (II) salts, which are usually soluble in water with green color, is carried out gravimetrically and qualitatively in the quantitative separation with dimethylglyoxime solution (Tschugajew's reagent). Nickel salts are previously optionally precipitated by ammonium sulfide as gray-black nickel (II) sulfide and dissolved in nitric acid. The specific detection is then possible by reaction with dimethylglyoxime in ammoniacal solution. The raspberry red bis (dimethylglyoximato) nickel (II) precipitates as a complex:
Since nickel is quantitatively precipitated from ammoniacal solution with dimethylglyoxime, this detection is also useful for quantitative gravimetric nickel analysis. From ammoniacal solution, a quantitative determination can also be made by means of electrogravimetry on a platinum mesh electrode. Similar to other heavy metals, nickel is today usually determined quantitatively by atomic spectroscopy or mass spectrometry in the ultratrace region as well. Extremely sensitive is the inverse voltammetry with adsorptive accumulation of the Ni-dimethyglyoxime complex on hanging drops of mercury or mercury film.
Connections
Nickel occurs in compounds mainly in the oxidation state + II. The levels 0, + I, + III and + IV are rare and usually unstable. Nickel forms a variety of mostly colored complexes.
Oxide
Nickel (II) oxide and nickel (III) oxide are green and black solids, respectively, and are used to make ceramics, glasses and electrodes. In addition, they are used as catalysts for the hydrogenation of organic compounds. Often, like many other binary metal oxides, nickel (II) oxide is not stoichiometric, meaning that the nickel-oxygen ratio deviates from 1: 1. This property is accompanied by a color change wherein the stoichiometrically correct nickel (II) oxide is green and the non-stoichiometric nickel (II) oxide is black. Nickel (III) oxide has a strong oxidizing effect and is unknown as a pure substance.
halides
Nickel (II) chloride is a yellow, highly hygroscopic solid which serves as a dye for ceramics and for the production of nickel catalysts. In addition to the anhydrous form, there are still hydrous nickel (II) chlorides, z. As the green nickel (II) chloride hexahydrate, which crystallized from aqueous nickel chloride solutions. The anhydrous nickel (II) chloride has a cadmium (II) chloride type trigonal crystal structure having the space group R3m (space group number 166). The hexahydrate crystallizes in the monoclinic crystal system in the space group C2 / m (space group No. 12).
Nickel (II) fluoride is also highly hygroscopic and forms yellowish to green tetragonal crystals. Unlike many fluorides, it is stable in the air. It crystallizes in the tetragonal crystal system with the space group P42 / mnm (space group number 136). The tetrahydrate crystallizes in the orthorhombic crystal system with the space group P21ab (space group number 29, position 3).
Other inorganic nickel compounds
Nickel (II) hydroxide and nickel (III) oxide hydroxide are used to store electrical energy in nickel-cadmium and other nickel accumulators.
Nickel (II) nitrate is used in the ceramics industry as a brown pigment, in dyeing as a mordant, for electrolytic nickel plating, for the recovery of nickel (II) oxide and for the production of pure catalyst nickel. Nickel (II) nitrate is a strong oxidizer and usually occurs in the form of its hexahydrate Ni (NO3) 2 · 6 H2O.
Nickel (II) sulfate and ammonium nickel (II) sulfate are used in plating (nickel plating). Nickel (II) sulfate is the most technically important nickel compound. It is used for the production of other nickel compounds and catalysts. The aqueous solutions of nickel (II) sulfate and nickel (II) chloride are used for the electrodeposition of metallic nickel layers. Furthermore, it is used in dyeing as a mordant and in the production of gas masks.
Nickel (II) carbonate occurs in several hydrate forms. It is used as a catalyst in the fat hardening and for the production of nickel (II) oxide, ceramic paints (pigments) and glazes and in electroplating. It formed a trigonal crystal system with the space group R3c (space group number 161).
Nickel (II) sulfide precipitates from ammoniacal, but not acidic, nickel containing solutions with ammonium sulfide. As a result, nickel can be separated with the ammonium sulfide group in the cation separation process.
Nickel Antimonide is a shiny metallic mineral and has a bright coppery red color. Nickel antimonide is used as a material in magnetic field plates where it is inserted between magnetically sensitive layers of indium antimonide. Magnetic field plates change their electrical resistance as a function of the magnetic flux density and serve as a sensor for magnetic fields. It forms a hexagonal crystal structure in the space group P63 / mmc (space group number 194).
Organic nickel compounds
Nickel tetracarbonyl Ni (CO) 4 is a colorless, very toxic liquid. It is an important intermediate in the moon process. Nickel tetracarbonyl was the first metal carbonyl compound discovered.
nickel complexes
Nickel and especially nickel (II) ions form many, mostly colored complexes. The coordination numbers 6, 5, or 4 are the most common. In the case of weak, monodentate ligands, for example water, they are usually present as octahedral and paramagnetic high-spin complexes with coordination number 6. Strong ligands such as cyanide form square-planar, diamagnetic low-spin complexes. Dimethylglyoxime also forms a square-planar complex, as the complex is additionally stabilized by hydrogen bonds. The latter bis (dimethylglyoximato) nickel (II) complex is important for the wet chemical detection of nickel. Anionic nickel complexes end in "-niccolate".
Examples of ammine complexes are the blue tetraammine nickel (II) and violet hexaammine nickel (II) complex. Both compounds are obtained by addition of ammonia to nickel (II) salt solutions:
Addition of potassium cyanide to nickel (II) salt solutions initially produces nickel (II) cyanide, which dissolves in excess of potassium cyanide to yellow potassium tetracyanoniccolate (II):
A corresponding compound is formed with potassium thiocyanate. A very sensitive compound is the potassium hexafluoroniccolate (IV) (K2 [NiF6]). Potassium tetracyanoniccolate (II) can be prepared from monovalent nickel by using a strong reducing agent K4 [Ni2 (CN) 6]. In addition, there are a variety of complexes with organic ligands such as ethylenediamine or anions of carboxylic acids.


![{\ displaystyle {\ ce {NiC2O4 -> [T] [] Ni + 2 CO2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4f5ac7119d30ce593cf99675097e91e39e33a04d)


![{\ displaystyle {\ ce {NiSO4 + 4NH4 + + 4OH- -> [Ni (NH3) 4] SO4 + 4H2O}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fed711e628c3ba92046eb178a459488074a035c9)
![{\ displaystyle {\ ce {Ni (CN) 2 + 2KCN -> K2 [Ni (CN) 4]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7a2b8181f20f5d9ad99c2900689b699739283d0d)
