Cadmium, cd, atomic number 48
Cadmium price, history, occurrence, extraction and use
Cadmium (also rarely cadmium, Greek καδμία kadmía, Latin cadmea, 'oxidic or carbonate-containing zinc earth') is a chemical element with the element symbol Cd and the atomic number 48. It is usually counted among the transition metals, although it has a closed d shell and thus more similar to the main group elements, especially the alkaline earth metals. In the periodic table it is in the 5. Period as well as the 2. Subgroup (group 12) or zinc group.
History
1817 independently discovered cadmium in contaminated zinc carbonate by Friedrich Stromeyer and Carl Samuel Hermann. Stromeyer noticed that contaminated zinc carbonate discolored on heating - a behavior that did not show pure zinc carbonate. For nearly 100 years, the metal was only won in Germany.
Pliny the Elder reported in his around the year 77 natural history Naturalis historia of Galmeifunden in Germania: "cadmea [...] ferunt nuper etiam in Germania provincia repertum" (German: "recently was found in the province of Germania Galmei"). The term cadmium was already used in the Middle Ages, probably for zinc or its carbonate ore. As evidenced by a document issued by Emperor Frederick II in April 1226 in Ravenna, this grants the right to the Benedictine monastery of St. Paul in Lavanttal "ut Cadmiae tam argentj quam plumbi et ferri, que in territorio ipsius monasteri de cetero inveniri contigerint "ad opus suum" (German: "that the zinc, as well as silver, as well as lead and iron, which is found in the area of the monastery, is used for its purpose").
Despite the toxicity of cadmium and its compounds, 1907's British Pharmaceutical Codex has been shown to use cadmium iodide as a treatment for scrubs (scrofulous glands) and chilblains (scrofulous glands).
1907 defined the Ångström as the 1 / 6438,4696 times the wavelength of a red spectral line of cadmium in dry air with a carbon dioxide content of 0,03% at a temperature of 15 ° C and a pressure of 1 atm. The 1960 General Conference on Weights and Measures accepted the 1.553.164,13-fold wavelength of a red spectral line of cadmium as the secondary definition of a meter.
1942 used Enrico Fermi cadmium sheets in the world's first nuclear reactor. The sheets could be pushed in and out of the reactor to control the chain reaction. Cadmium can capture moderated gap neutrons and thus influence the criticality of the reactor.
occurrence
Cadmium is a very rare element. Its share in the earth's crust is only about 3 · 10−5 . Cadmium occurs extremely seldom in solid form, i.e. in elemental form. So far, only five sites in three countries are known: the Khann'ya River in the Vilyui Basin, the Jana River Basin near Verkhoyansk and the Billeekh Intrusion in the Russian Republic of Sakha (Yakutia, Eastern Siberia); the Goldstrike pits near Lynn in Eureka County, Nevada, and the Burabaiskii massif in the Aqumola area of Kazakhstan.
As cadmium-containing ores are mainly the cadmium screen Greenockit (CdS) with up to 77,81% Cd and the Cadmiumspat Otavit (CdCO3) with up to 65,20% Cd known, but which are too rare for commercial degradation. Both are almost always associated with various zinc ores such as sphalerite (ZnS) and smithsonite (ZnCO3).
Altogether so far (stand 2018) something more than 20 cadmium minerals are known. The very rare cadmium oxide Monteponite has the highest Cd content up to 87,54%. Other minerals include Hawleyit (77,81% Cd), Cadmoselite (58,74% Cd) and Drobecit (IMA 2002-034, 40,07% Cd).
Cadmium as a mineral
Naturally occurring cadmium in its elemental form was first described by 1979 BV Oleinikov, AV Okrugin and NV Leskova. and recognized by the International Mineralogical Association (IMA) as an independent mineral species (IMA Internal Accession Number: 1980-086a).
According to the classification of minerals according to Strunz (9 edition) cadmium is under the system no. 1.AB.05 (Elements - Metals and Intermetallics - Zinc-Brass Family - Zinc Group). In the outdated 8. However, cadmium is not yet listed in the edition of Strunz's Mineral Systematics. Only in the last 2018 updated "Lapis mineral directory", which is based on this form of system numbering out of consideration for private collectors and institutional collections, the mineral received the system and mineral no. I / A.4-40. The classification of minerals according to Dana, which is predominantly used in English-speaking countries, leads the element mineral under the system no. 01.01.05.02.
Extraction and presentation
Cadmium is obtained exclusively as a by-product of zinc smelting and, to a lesser extent, of lead and copper smelting. Smaller quantities also apply to the recycling of iron and steel.
The production of cadmium depends on how the zinc is extracted. In dry zinc extraction, the cadmium is first reduced with the zinc. Because cadmium has a lower boiling point than zinc, it evaporates more easily. As a result, a cadmium-zinc mixture evaporates from the reduction vessel and reacts elsewhere with oxygen to cadmium and zinc oxide. Subsequently, this mixture is mixed in a distillation vessel with coke and distilled off the cadmium from the zinc. Fractional distillation allows higher levels of cadmium to be achieved.
During wet zinc extraction, the dissolved cadmium ions are reduced and precipitated with zinc dust. The resulting cadmium is oxidized with oxygen to cadmium oxide and dissolved in sulfuric acid. From the resulting cadmium sulfate solution is obtained by electrolysis with aluminum anodes and lead cathodes particularly pure electrolyte cadmium.
Physical Properties
Cadmium is a silvery shiny metal with a density of 8,65 g / cm3. It is soft (Mohs hardness 2), plastically deformable and can also be cut with a knife as to draw wires and hammer into leaflets.
Cadmium solidifies exclusively in the hexagonal crystal system in the space group P63 / mmc (space group number 194). The lattice parameters of pure cadmium are a = 0,2979 nm (corresponds to 2,98 Å) and c = 0,5617 nm (corresponds to 5,62 Å) for 2 formula units per unit cell.
Similar to tin, typical noises occur when bending cadmium of medium purity (called pewter tin in tin). Polished cadmium loses its shine in air after a few days, even if it is more resistant to corrosion than zinc. In carbonated air, it forms a greyish white, carbon dioxide-containing coating. Heavily heated, it burns with a reddish to yellow flame to brownish cadmium oxide cadmium dioxide.
CdO was tested for its high toxicity during World War II by the United States for its suitability as a chemical warfare agent.
Chemical properties
In chemical compounds it is usually divalent. It is chemically similar to zinc, but tends to form complex compounds with the coordination number 4. In the air cadmium forms a darkening of the surface due to the oxidation. In alkaline medium, the surface is insoluble, heavy in sulfuric acid and hydrochloric acid, and readily soluble in nitric acid.
Usage
Because of the high toxicity of cadmium its importance decreases. Since December 2011 it is banned in jewelry, alloys for soldering and in PVC in the European Union. Cadmium is or has been used:
- as corrosion protection for ferrous materials (cadmium plating)
- as surface coating for aluminum materials in military engineering (eg rocket launchers)
- for nickel-cadmium batteries
- for yellow to deep red color pigments of cadmium sulfide and cadmium selenide for paints and plastics (meanwhile low practical importance because of possible health hazards, especially when burning corresponding articles)
- as alloying metal in low-melting alloys, for example bearing materials or Wood's metal
- earlier than lubricant in disc brakes
- as a component of soldering materials (solder), also for brazing alloys
- for the production of semiconductors
- Cadmium oxide as a phosphor in black-and-white television tubes and addition in blue and green phosphor of color tubes
- Cadmium oxide as an admixture to silver in switching contacts
- as a shielding material against thermal neutrons and for control rods in nuclear technology due to the particularly high cross section of the 113 isotope for neutron capture
- as a source of high-energy gamma radiation (around 7 MeV) from thermal neutrons for the later generation of positrons by pair generation
- Cadmium sulfide in exposure meters whose spectral sensitivity is similar to that of the human eye
- Cadmium telluride as an infrared-sensitive sensor for cameras (focal plane arrays)
- in thin-film solar cells as cadmium telluride or cadmium sulfide for power generation
- Cd stearate as a stabilizer in plastics, for example in PVC (insensitive to light, but in the meantime of low practical importance because of possible health hazards)
- earlier in the Weston Normal Elements to determine the unit of measurement of voltage, 1 volts
- Cadmium bismuth alloys for fuses
- Silver-cadmium alloys as a deoxidizer in the production of sterling silver
- for jewelery: gold-green gold-cadmium alloys
- Cadmium lamp
- Helium-cadmium lasers
- Cadmium ions to block voltage-activated calcium channels in electrophysiology
for coloring glass in yellow, orange and red by adding cadmium sulfide, selenide and telluride or mixtures thereof.
The cadmium chalcogenides cadmium sulfide (yellow), cadmium selenide (red), and cadmium telluride (black) are important II-VI semiconductors. They are produced, for example, nanoparticulate as quantum dots (quantum dots) and used, inter alia, in biochemistry in vitro.
proof
As a preliminary sample for cadmium, the so-called Glühröhrchenprobe can serve. For this purpose, some stock substance is heated in a high-melting Glühröhrchen and reduced the resulting sulfide-oxide mixture with sodium oxalate to the metals. As a volatile component, cadmium evaporates and deposits as a metal mirror on the upper part of the tube.
By subsequent addition of sulfur and renewed annealing, cadmium sulfide forms from the metal mirror and sulfur vapor, which is red in the heat and yellow at room temperature. This color change can be repeated a few times.
The detection reaction for cadmium cations is precipitation with sulfide solution or hydrogen sulfide-water as yellow cadmium sulfide. Other heavy metal ions interfere with this detection, so that before a cation separation is carried out.
For the quantitative determination of cadmium traces the polarography is recommended. Cadmium (II) ions enter a level in 1 M KCl at -0,64 V (vs. SCE). In the ultra-trace range, inverse voltammetry can be used on mercury electrodes. Very sensitive is also the graphite tube AAS of cadmium. Here, 0,003 μg / l can still be detected. The relatively volatile element does not tolerate a high pyrolysis temperature. A matrix modifier such as palladium magnesium nitrate can help.
safety instructions
Cadmium is very toxic and its compounds are classified as harmful (such as cadmium telluride), toxic (eg cadmium sulphide) or very toxic (cadmium oxide); there is also a reasonable suspicion of a carcinogenic effect in humans. Ingested cadmium-containing dust causes damage to the lungs, liver and kidneys.
In working areas where heated cadmium compounds are used (soldering stations and cadmium baths), ensure good ventilation or extraction. According to the Chemical Interdiction Ordinance, the cadmium content in plastics must not exceed 0,01 percent by weight (100 mg / kg). This limit applies throughout the European Union.
Since December 2011 applies a stricter ban on cadmium in plastics, paints, stabilizers and certain metal processing under the REACH Regulation, entry 23 in Annex XVII. Previously, silver brazing typically contained 10% to 25% cadmium, children's jewelry up to 30%, PVC 0,2%. With the 10. In December 2011, the ban on cadmium-containing solders in soldering and the production and marketing of cadmium-plated jewelry was extended. In addition, all PVC-containing products, with the exception of PVC recycling, were also included. With the Regulation (EU) 2016 / 217 of the 16. In February 2016, cadmium in certain paints and varnishes was added to the 23 entry in Annex XVII of the REACH Regulation. In addition, the cadmium content in certain paints and varnishes containing zinc or products containing it has been limited. There are exceptions, for example because of the high power density of Ni-Cd batteries in cordless electrical appliances.
toxicology
Cadmium is an unavoidable by-product of zinc, lead and copper extraction in the chemical industry. Cadmium can also be found in fertilizers and pesticides.
Recording and dangers
The World Health Organization has repeatedly downgraded its statement on tolerable intake of cadmium in recent years, most recently 2013 to a tolerable monthly intake (TMI) of 25 μg per kilogram of body weight. The European Food Safety Authority has issued 2009 with a significantly lower level of 2,5 μg per kilogram body weight tolerable weekly intake (TWI).
Cadmium is absorbed by humans mainly through food. The cadmium rich foods include: liver, mushrooms, shellfish and other shellfish, cocoa powder and dried seaweed. In addition, flaxseed contains a lot of cadmium, which is why it is recommended to take no more than 20 g of flaxseed daily. In addition, since the introduction of artificial fertilizers, there has been an accumulation of cadmium on agricultural land and thus in almost all foods. The resources of phosphates are limited, and most resources are contaminated with cadmium or radioactive heavy metals. The cadmium content of the phosphate deposits is very different. Many industrialized countries have already introduced a limit on cadmium in fertilizers. In Austria the cadmium concentration is limited to 75 mg / kg P2O5. Also, tobacco smoke transports relatively large amounts of cadmium into the lungs, from where it spreads with the blood in the body.
Especially people working in high-cadmium factories are at increased risk. But there are also dangers from wild dumps and metal works. Inhaling cadmium can seriously damage the lungs and even lead to death. Accidents in industry - such as in the Chinese province of Guangdong - and decades of emissions - such as in the case of Itai Itai disease (in humans) and Gressenicher disease (in grazing livestock) - make the real dangers clear.
Damage in humans
Cadmium can gradually accumulate in the body due to industrial or environmental reasons and cause a chronic poisoning that is difficult to recognize.
Cadmium is absorbed from the diet to approximately 5% in the intestine. With iron and calcium deficiency, the absorption rate increases, suggesting that all three metals use the same transport pathway. Cadmium first stimulates in the liver the synthesis of metallothioneins, with which it forms a complex and is transported via the bloodstream to the renal glomeruli where it is filtered and resumed from the renal tubules. In the tubule cells, the metallothionein-cadmium complex is metabolized and Cd is released. Here again, cd activates an increased synthesis of metalthione, which binds even more cadmium. Accumulation in the kidneys causes damage to this organ, resulting in proteinuria. Due to this protein binding cadmium is excreted only extremely slowly, the half-life for the body remains up to 30 years. Therefore, the cadmium content increases from birth and falls again at an age of 50-60 years.
Cadmium also damages the bones, as it ultimately leads to the mobilization of calcium. Cd competes in the intestine with the calcium for the binding sites on the Ca-binding protein in the intestinal mucosa. In addition, Cd blocks the re-synthesis of 1,25-dihydroxycholecalciferol (calcitriol) in kidney tubulus cells. 1,25-Dihydroxycholecalciferol is necessary to activate the synthesis of the calcium-binding protein in the intestinal mucosal cell. In summary, cadmium causes a reduced reabsorption of calcium in the intestine and kidney, as well as the increased excretion with urine with the consequence of a calcium release from the bones and thus the degradation of the same.
In the case of acute cadmium poisoning, biliary excretion may be assisted by the administration of penicillamine or dimercaprol. An effective, beyond treatment of acute cadmium poisoning is not known.
Symptoms
- Diarrhea, stomach ache and violent vomiting
- kidney damage
- bone fractures
- Damage to the central nervous system
- Damage to the immune system
- Disorders of reproduction and possibly even infertility
- Mental disorders
- Possible DNA damage and carcinogenesis
- Loss of the sense of smell
Connections
→ Category: Cadmium compound
Oxides and hydroxides
- Cadmium oxide CdO
- Cadmium hydroxide Cd (OH) 2
halides
- Cadmium fluoride CdF2
- Cadmium chloride CdCl2
- Cadmium bromide CdBr2
- Cadmium iodide CdI2
chalcogenides
- Cadmium sulfide CdS
- Cadmium selenide CdSe
- Cadmium telluride CdTe
Other compounds
- Cadmium sulphate CdSO4
- Cadmium nitrate Cd (NO3) 2
- Cadmium cyanide Cd (CN) 2
- Cadmium Stearate Cd (C17H35COO) 2
Cadmium Price -> Strategic Metals Prices
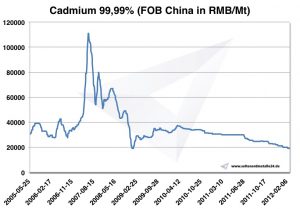
Chart Cadmium 2005-2012


