Zirconium, Zr, atomic number 40
Zirconium price, occurrence, extraction and use
Zirconium, often too zirconium, is a chemical element with the element symbol Zr and the ordinal number 40. Its name derives from zircon, the most common zirconium mineral. In the periodic table it is in the 5. Period; it is the second element of 4. Group (obsolete 4 subgroup) or titan group. Zirconium is a very corrosion resistant heavy metal. Biological functions are unknown; it is present in small amounts (4 mg / kg) in the human organism and is not toxic.
The important zirconium-containing mineral zircon (Zr [SiO4]) has been known as a gemstone since ancient times. Zirconium as an element was discovered in 1789 by Martin Heinrich Klaproth in a sample of the mineral zircon from Ceylon and named after it. The metal was first presented in 1824 by Jöns Jakob Berzelius through a reduction of K.2ZrF6 with potassium. To do this, he heated "A mixture of hydrofluoric zirconium potash with potassium in an iron tube". After treatment with water, drying and prolonged heating with dilute hydrochloric acid, Berzelius received a "Lumpy powder that looks like coal black" was and only "By squeezing with the polishing steel a dark gray color and shine" received. The correct atomic mass, on the other hand, could not be determined until 1924 because - besides errors in the implementation of the experiments - it was not known that zirconium always contains small amounts of hafnium. Without this information, measurements always gave a slightly too high atomic mass. The first practical application of zirconium was as a smokeless flash powder.
occurrence
Zirconium occurs in the earth's crust with a content of about 0,016%. In the frequency ordered list of elements, zirconium is on 18. Place and is more common than the more familiar elements of chlorine and copper. Although it is very widespread, it is usually found only in very small quantities and in very small crystals (typically around 0,1 mm). Therefore, zirconium has been considered rare in ancient times. Zirconium is found mainly in silicate intrusive rocks such as granite. It does not come dignified, but only in some minerals, especially as zircon (ZrSiO4) and Baddeleyit (ZrO2) and the rarer red eudialyte (Na4(CaCeFeMn)2ZrSi6O17(OHCl)2). It is almost always associated with hafnium. Due to its high melting point of 2550 ° C, its high hardness and low reactivity, zirconium is the oldest mineral that can be found on Earth and can be used for radiometric age determinations based on embedded uranium and thorium isotopes.
Secondary deposits, so-called soap deposits, are usually used as raw materials. These arise when the surrounding rock is weathered and only the particularly weather-resistant zircon remains. Other such deposits can arise from water currents that wash out the zirconium crystals and wash them up in other places. Primary deposits, on the other hand, usually have a zirconium content that is too low for profitable mining.

Temporal development of zircon promotion
The most important zirconium deposits are in Australia, the USA and Brazil. With recoverable reserves of 38 million tons, the world annual production of zirconium minerals 2006 was at 920.000 tonnes (calculated as zirconium). Of these, only about 5% are processed to metal and alloys. The main producing countries were 2006 by far Australia and South Africa.
Extraction and presentation
Zirconium, the most common zirconium raw material, must first be converted into zirconium dioxide before further processing. For this, the zircon is boiled in a sodium hydroxide melt (alkaline digestion). The zirconia is then reacted with coke in the arc to form zirconium carbonitride (carbon and nitrogen-containing zirconium) and then with chlorine to form zirconium tetrachloride.

A direct reduction of zirconium dioxide with carbon (as in the blast furnace process) is not possible, since the carbides formed are very difficult to separate from the metal. Instead, zirconium tetrachloride is reduced to zirconium metal in the so-called Kroll process with magnesium in a helium atmosphere.

The Van Arkel de Boer process is used to obtain purer zirconium. During heating under vacuum, the zirconium initially reacts with iodine to form zirconium (IV) iodide. This is broken down again to zirconium and iodine on a hot wire:

Zirconium tetraiodide is formed from zirconium and iodine at 200 ° C; it disintegrates again at 1300 ° C.
Zirconium and hafnium can not be separated in a simple chemical way. Therefore, even this high purity zirconium still contains hafnium. Since it is important for many applications in reactor technology that the zirconium no longer contains hafnium, separation processes for these two metals play an important role. One possibility is extraction methods in which the different solubility of zirconium and hafnium compounds in special solvents is exploited. Frequently, the thiocyanates and their different solubility in methyl isobutyl ketone are exploited. Other possibilities are ion exchangers or the fractional distillation of suitable compounds.
Features
Physical Properties

Crystal structure of α-zirconium
Zirconium is a silvery shiny heavy metal (density 6,501 g / cm3 at 25 ° C), it externally resembles steel. The metal crystallizes in two different modifications in which it can be converted by temperature change. Below 870 ° C crystallizes α-zirconium in the hexagonal crystal system (hexagonal-dense sphere packing, magnesium type) in the space group 6/ mmm with the grid parameters a = 323 pm and c = 514 pm as well as two formula units per unit cell. At 870 ° C, the crystal structure changes to the cubic-centered β structure (tungsten type) with the space group  and the lattice parameter a = 361 pm.
and the lattice parameter a = 361 pm.
Zirconium is relatively soft and flexible. It can be easily processed by rolling, forging and hammering. However, it becomes brittle and difficult to process due to low levels of hydrogen, carbon or nitrogen contamination in the metal. The electrical conductivity is not as good as that of other metals. It is only about 4% of that of copper. In contrast, zirconium is a good conductor of heat. Melting point and boiling point are slightly higher in comparison with the lighter homologue titanium (melting point: titanium: 1667 ° C, zirconium: 1857 ° C). Also, the electrical and thermal conductivity are better. Below 0,55 K, zirconium becomes superconducting.
The properties of zirconium and the heavier homologue hafnium are very similar due to the lanthanide contraction. This requires similar atomic radii (Zr: 159 pm, Hf: 156 pm) and thus similar properties. The two metals differ considerably in their density (Zr: 6,5 g / cm3, Hf: 13,3 g / cm3).
An important property, because of which zirconium has gained great importance in reactor construction, is its small capture cross-section for neutrons. In this capacity, zirconium is also very different from hafnium. This makes the complex separation process necessary for these applications.
Chemical properties
Zirconium is a base metal that reacts with many non-metals, especially at high temperatures. Mainly as a powder, it burns with a white flame to form zirconium dioxide, in the presence of nitrogen also to zirconium nitride and zirconium oxynitride. Compact metal only reacts with oxygen and nitrogen when it is white heat. At increased pressure, zirconium reacts with oxygen even at room temperature, since the zirconium oxide formed is soluble in the molten metal.
Zirconium is passivated in the air by a thin, very dense layer of zirconium oxide and is therefore inert. It is therefore insoluble in almost all acids, only aqua regia and hydrofluoric acid attack zirconium at room temperature. Aqueous bases do not react with zirconium.
isotope
There are many isotopes of the zirconium between 78Zr and 110Zr known. Natural zirconium is a mixed element consisting of five isotopes. these are 90Zr, which occurs most frequently with a share of 51,45% of natural zirconium, as well as the heavier isotopes 91Zr (11,32%), 92Zr (17,19%), 94Zr (17,28%) and 96Zr with 2,76% share. 96Zr is the only natural isotope that is weakly radioactive; it decays with a half-life of 24 · 1018 Years under double beta decay 96Mo. The isotope 91Zr can be detected with the aid of NMR spectroscopy.
Usage
 An important use for zirconium are Zircaloy's uranium fuel cell shells in nuclear power plants. This alloy consists of approximately 90% zirconium and small amounts of tin, iron, chromium or nickel, but must not contain hafnium. The reason for choosing this element is the already described low capture cross section for thermal neutrons with simultaneously high corrosion resistance, which makes it also suitable as a building material for chemical plants, especially for special apparatus parts such as valves, pumps, pipes and heat exchangers. As an alloying addition to steel, it also increases corrosion resistance. Surgical instruments are manufactured from appropriate alloys.
An important use for zirconium are Zircaloy's uranium fuel cell shells in nuclear power plants. This alloy consists of approximately 90% zirconium and small amounts of tin, iron, chromium or nickel, but must not contain hafnium. The reason for choosing this element is the already described low capture cross section for thermal neutrons with simultaneously high corrosion resistance, which makes it also suitable as a building material for chemical plants, especially for special apparatus parts such as valves, pumps, pipes and heat exchangers. As an alloying addition to steel, it also increases corrosion resistance. Surgical instruments are manufactured from appropriate alloys.
Since zirconium reacts with small amounts of oxygen and nitrogen, it can be used as a getter material in incandescent lamps and vacuum systems to maintain the vacuum. This property is also used in metallurgy to remove oxygen, nitrogen and sulfur from steel.
Because of its ability to emit a very bright light when burned, it was used in addition to magnesium as a flash powder. Unlike magnesium, zirconium has the advantage of being smoke-free. This feature is also exploited in fireworks and signal lights.
Zirconium emits a surge of sparks when it hits metal surfaces and is flammable. The military uses this in some types of ammunition, such as the special shotgun ammunition Dragon's Breath and the US-American all-purpose glide bomb AGM-154 JSOW. In film technology, this effect is used for non-pyrotechnic impact effects of, for example, bullets on metal surfaces.
Zirconium-niobium alloys are superconducting and remain so even when strong magnetic fields are applied. They are therefore used for superconducting magnets.
In addition to aluminum-containing alums, zirconium salts are used in the "white tanning" of skins.
safety instructions
There are no known toxic effects of zirconium and its compounds. Because of the dense oxide layer, compact zirconium is not flammable. In powder form, on the other hand, it may start to burn when heated in air. Zirconium fires are very dangerous as they can not be used for extinguishing water (vigorous reaction with hydrogen formation), nor carbon dioxide or halon. Zirconium fires must be extinguished with metal fire extinguishers (class D) or dry sand.
proof
With Alizarin Red-S, zirconium acid forms a characteristic red-violet compound (colored lake), which disappears upon addition of fluoride ions to form the zirconium fluoro complex. This reaction can serve as qualitative detection of both zirconium and fluorine. Since even small amounts of fluoride (and other anions) interfere, this detection is unsuitable for mineral analysis. In addition, some other organic compounds, such as tannin, Kupferron, phenylarsonic acid, oxine or xylenol orange, are suitable as a detection reagent. Another characteristic compound is zirconium chloride ZrOCl2 · 8 H2O, which crystallizes in typical needles. In modern analysis, zirconium can be detected by atomic absorption spectrometry (AAS) or mass spectrometry (also by the isotope pattern).
One possibility for quantitative analysis is the precipitation of sparingly soluble zirconium hydroxide with ammonia and subsequent calcination to zirconia.

- Precipitation of the hydroxide
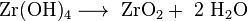
- Transfer to the weighing mold
Connections
As a base metal, zirconium forms a multitude of compounds. Most zirconium compounds are salts. They are often very stable and have a high melting point. The + IV oxidation state is preferred and the most stable. But there are also compounds in the oxidation states + III to + I, and in complexes even in the states 0, −I and −II.
zirconia
 The most important zirconium compound is zirconium dioxide ZrO2, a very stable and refractory oxide. Zirconium dioxide is used to produce refractory linings in crucibles and ovens. In order to use it, however, it must be stabilized with calcium, yttria or magnesia to stabilize the cubic high temperature phase. In addition, it is used as an abrasive and because of the white color as a white pigment for porcelain.
The most important zirconium compound is zirconium dioxide ZrO2, a very stable and refractory oxide. Zirconium dioxide is used to produce refractory linings in crucibles and ovens. In order to use it, however, it must be stabilized with calcium, yttria or magnesia to stabilize the cubic high temperature phase. In addition, it is used as an abrasive and because of the white color as a white pigment for porcelain.
Zirconium dioxide crystals are colorless and have a high refractive index. That is why they are used under the name zirconia as an artificial gemstone and substitute for diamonds.
If zirconium oxide is mixed with yttrium oxide, further application possibilities arise. At three percent yttrium oxide content, the ZrO2 stabilized in a distorted fluorite structure. As a result, it acts as a conductor for oxygen ions at temperatures above 300 ° C. An important application for this is the Lambda probe in cars, which is used to measure the oxygen content in exhaust gases for the catalyst. At 15% yttria content, zirconia emits a very bright, white light at 1000 ° C. This is used in the so-called Nernst lamp application. Since yttrium-zirconium ceramics have an extremely high fracture toughness, they are used, for example, in dentistry as a highly stable crown and bridge framework, in artificial hip joints and dental implants or as a connecting element in telescopes. In the process, they are increasingly replacing gold and other metals in their function.
Zirconia is also often used for ball bearings. Especially for the bearing races, ZrO2 the great advantage that the coefficient of thermal expansion is close to that of steel. Other technical ceramics such as silicon nitride usually have a considerably lower coefficient of thermal expansion.
halides
With the halogens fluorine, chlorine, bromine and iodine, zirconium forms several series of compounds. All halogens are compounds of the form ZrX4, ZrX3 and ZrX2 known. In addition there are the chlorides, bromides and iodides of the form ZrX. The tetrahalides of the form ZrX are the most stable4, None of the zirconium halides are known to have important applications, with zirconium chlorides being intermediates in the preparation of pure zirconium.
Further zirconium compounds
Zirconium silicate, ZrSiO4, better known under the mineral name zircon, is the most common zirconium compound found in nature. It is the most important source of zirconium and its compounds. Zircon is also used as a gemstone.
Organic zirconium compounds are mostly unstable. Organic zirconium complexes, so-called. zirconocenes, with radicals such as cyclopentadienyl. They are technically important as a catalyst in the polymerization of alkenes, especially for the production of polypropylene. Another application of an organic zirconium compound is in the hydrozirconation, This alkenes are using the Schwartz reagent Cp2ZrHCl (Cp = cyclopentadienyl) converted into alcohols or halogenated hydrocarbons. In the reaction of terminal alkynes with the Schwartz reagent arise in the hydrozirconation trisubstituted double bonds, the further reaction with an electrophilic reagent leads to trans-functionalized alkenes in high stereochemical purity.
Aluminum-zirconium complexes can be used as antiperspirant.
Potassium hexafluoridozirconate (IV) K2ZrF6 (CAS: 16923-95-8) can be used to separate zirconium from hafnium.
Zirconium carbonate exists as a basic complex. It is used, among other things, in the paper industry.
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, atomic number | Zirconium, Zr, 40 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 4, 5, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-67-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth shell | 0,021% | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| atomic mass | 91,224 u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 155 (206) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 148 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electron configuration | [Kr] 4d2 5s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. ionization | 640,1 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. ionization | 1270 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. ionization | 2218 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. ionization | 3313 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | fixed | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| modifications | two (α- / β-Zr) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| crystal structure | hexagonal; cubic> 1140 K (867 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 6,501 g / cm3 (25 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( = 1,1 10−4) = 1,1 10−4) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| melting point | 2130 K (1857 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 4682 K (4409 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 14,02 · 10−6 m3/ mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 590,5 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| heat of fusion | 16,9 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| vapor pressure | 0,00168 Pa at 2125 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| speed of sound | 4650 (long.), 2250 (trans.) M / s at 293,15 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 270,0 J / (kg · K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 2,36 · 106 A / (V · m) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thermal conductivity | 22,7 W / (m K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| oxidation states | 4, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| normal potential | −1,553 V (ZrO2 + 4 H.+ + 4 e- → Zr + 2 H2O) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electronegativity | 1,33 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| isotope | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
Zirconium prices
Zirconium price -> prices for strategic metals


