Silicon, Si, atomic number 14
Silicon price, occurrence, extraction and use
Silicon, also silicon, is a chemical element with the symbol Si and the atomic number 14. It's in the 4. Main group (carbon group), or the 14. IUPAC Group, and the 3. Period of the Periodic Table of the Elements. In terms of mass fraction (ppmw), it is the second most abundant element in the earth's shell after oxygen.
Silicon is a classic semi-metal, therefore has both properties of metals and non-metals and is an elemental semiconductor. Pure, elemental silicon has a gray-black color and has a typically metallic, often bronze to bluish luster.
Silicon is extremely important for the electronics and since 2018 in isotope-pure form also serves for the definition of the kilogram. Elemental silicon is non-toxic to the human body, silicon in bound silicate form is important for humans. The human body contains about 20 mg / kg of body mass of silicon; the amount decreases with age.
Spelling and etymology
By default, the element 'silicon' is written. The notation with, c 'is mainly used in the chemical jargon. Both spellings originate from the Latin term silicia, silica ', associated with Latin silex' pebble ',' rock '.
The English word for silicon is silicon. It is included, for example, in the name Silicon Valley. The occasionally encountered translation of silicone is a false friend, because silicones are a class of chemical compounds of silicon.
History
Use in pre-industrial times
Silicon compounds, especially rocks, have traditionally played an important role in human history as a building material. A typical example of an early stone structure is Stonehenge. Another important siliceous material that has been used as a building material for a long time is loam, which was initially used in branch-fiber clay construction, later in brick form. Cement, which is also silicate-containing, was first developed by the Romans.
Due to their sharp edges, siliceous rocks were also used as tools in the Stone Age. Obsidian, for example, was already mined as a particularly suitable tool material in the protohistoric period and widely distributed by commerce. Feuerstein was mined in chalk areas, such as Belgium and Denmark. In metal extraction, especially in steelmaking, silicate slag is used to protect the stoves and furnaces from oxygen ingress and as clay or sand molds; possibly the glassmaking was discovered.
Discovery as an element
For the first time in the year 1789 Antoine Lavoisier predicted that Silex is the oxide of a metal. In the year 1807, Humphry Davy postulated electrochemical tests for the existence of the metals silicon, aluminum, zirconium and glucinium (beryllium).
"Had I been so fortunate as to obtain this subject, and to have procured the metallic substances I was in search of, I should have proposed for the names of silicium, alumium, zirconium and glucium."
"If I'd been so happy to have more reliable evidence on the subject and to have taught the metallic substances I sought, I would have suggested for them the names silicon, alumium, zirconium, and glucium."
- Humphry Davy
In 1811, the chemist Joseph Louis Gay-Lussac and Louis Jacques Thénard (see Thénards Blau) produced impure and amorphous silicon (a-Si, the non-crystalline, allotropic form of silicon). To do this, they reacted silicon tetrafluoride with elemental potassium. A similar procedure was followed by Xnumx by Jöns Jakob Berzelius in Sweden by reacting a hexafluorosilicate with elemental potassium. Berzelius cleaned the resulting amorphous silicon by washing. He was the first to recognize the elemental nature of silicon and gave it its name.
The term silicon is derived from the Latin word silex (pebble, flint). He expresses that silicon is more common in many minerals.
The English term silicon was proposed to 1817 by the Scottish chemist Thomas Thomson (1773-1852). The ending -on is intended to indicate the chemical relationship to the non-metals carbon (carbon) and boron (boron).
The first production of pure, crystalline silicon in the year 1854 succeeded to the French chemist Henri Etienne Sainte-Claire Deville by means of electrolysis.
occurrence
The entire earth is about 15 mass percent of silicon; in particular, the mantle is composed to a considerable extent of silicate rock melts. The earth's crust is about 25,8 weight percent of silicon; this makes it the second most abundant chemical element after oxygen. Here, silicon occurs essentially in the form of silicate minerals or as pure silica.
So sand consists mainly of silica. Quartz is pure silica. Many gems are made of silicon dioxide and more or less admixtures of other substances, such as amethyst, rose and smoke quartz, agate, jasper and opal. Silicon forms silicates with many metals. Examples of siliceous rocks are mica, asbestos, clay, shale, feldspar and sandstone. The oceans also represent a huge reservoir of silicon: in the form of monomeric silicic acid, it is dissolved in considerable quantities in all oceans. Altogether (as of: 2011) 1437 silicon minerals are known, with the rare moissanite with a content of up to 70% having the highest silicon content (for comparison: mineral quartz has a silicon content of up to 46,7%).
Since silicon occurs in nature also in dignified, that is elementary form, it is recognized by the International Mineralogical Association (IMA) as a mineral and is in the Strunz'schen Mineral classification (9 edition) under the system no. 1.CB.15 (8 edition: I / B.05-10) led in the department of semi-metals and non-metals. In the mostly known in English-speaking classification of minerals according to Dana, the element mineral carries the system no. 01.03.07.01.
Solid silicon has been detected (2011) at 15 sites, including for the first time in the Nuevo Potosí deposit in Cuba. Other localities are in the People's Republic of China, Russia, Turkey and the United States.
Silicatkreislauf
Silicatic minerals are permanently degraded by reaction with the carbonic acid of the water to form metasilicic acid and carbonates, as shown by the example of calcium silicate:
The insoluble metasilicic acid further reacts with carbonic acid to form soluble orthosilicic acid:
However, orthosilicic acid reacts with itself again relatively quickly to form (amorphous) silicon dioxide and water, provided the pH value is ≥ 3. The absolute concentration of orthosilicic acid is relatively low (e.g. <approx. 7 mmol in sea water).
Incorporation of silica or water-soluble silicates in marine organisms (1.), Which sediment upon extinction after being drowned, or by volcanism and magma leakage on the seabed regresses the silicate minerals (2.) And the cycle is closed:
The time horizon in which this process takes place is several million years, so it is considerably longer than in the case of the carbon cycle of living nature.
Silicon in the living nature
In addition to the already mentioned essential nature of silicon, there are a number of organisms that produce structures containing silicon dioxide. The most well-known of these are the diatoms, sponges (Porifera, Spongiaria) and radiolarians, which form an exoskeleton of silicon dioxide by enzyme-catalyzed condensation of orthosilicic acid Si (OH) 4. Many plants also contain silica in their stems and leaves. Well-known examples here are the horsetail and the bamboo plant. The built-up silica backbone gives them additional stability.
Physiological significance for humans
Silicon seems to be needed for bone formation and maturation. In calves, the administration of orthosilicate led to the proliferation of collagen in the skin and cartilage. The desirable intake derived from animal experiments is 30 mg / d. Deficiencies in humans are not yet known.
Silica or Silica terra are oral products. Essentially, they contain silicic anhydrides (silicon dioxide) and are said to strengthen the skin, nails, bones and connective tissue and keep them healthy. An effect has not been scientifically proven.
An excess of silicon can cause erythrocyte hemolysis and, as a direct consequence, cell changes.
Extraction in the laboratory
Elemental silicon can be obtained on a laboratory scale by reduction, starting from silica or silicon tetrafluoride, with base metals. Reaction 2.) Is an aluminothermic process, which works only with addition of elemental sulfur, the third route corresponds to the element discovery:
Highly reactive amorphous silicon can be obtained by reduction with sodium or acidolysis of silicides:
Extraction in the industry
Elemental silicon is used in different degrees of purity in metallurgy (ferrosilicon), photovoltaics (solar cells) and in microelectronics (semiconductors, computer chips). Accordingly, it is common in the industry to classify elemental silicon based on different degrees of purity. A distinction is made between simg (metallurgical grade, crude silicon, 98-99% purity), sisg (solar grade, solar silicon, impurities smaller than 0,01%) and victory (electronic grade, semiconductor silicon, small 10-9 impurities). For solar cells, the purity of the material throughout its thickness is important to ensure the longest possible carrier lifetime, for many applications in microelectronics only the upper layers of about 20 to 30 microns must be highly pure.
Traditionally, the Siemens process is used, in which the silicon is first reacted with gaseous hydrogen chloride at 300-350 ° C in a fluidized bed reactor to trichlorosilane (silicochloroform).
After several distillation steps, the trichlorosilane is thermally decomposed in the presence of hydrogen in a reversal of the above reaction on heated hyperpure silicon rods at 1000-1200 ° C. The elemental silicon grows on the rods. The liberated hydrogen chloride is returned to the circulation. As a by-product, silicon tetrachloride precipitates, which is either converted to trichlorosilane and returned to the process or burned in the oxygen flame to pyrogenic silica. The Siemens process produces 19 kg of waste and by-products per kg of hyperpure silicon.
raw silicon
On an industrial scale, elemental silicon is obtained by reducing silica with carbon in the smelting reduction furnace at temperatures of about 2000 ° C. Starting material is quartz sand or quartz gravel.
2002 produced about 4,1 million tons of this industrial raw silicon (Simg). It is sufficiently clean for metallurgical purposes and is used as an alloying element and deoxidant for steels (improvement of corrosion resistance, cementite suppression) and as a raw material for silane production via the Müller-Rochow process, which ultimately serves mainly for the production of silicones. For the production of ferrosilicon for the steel industry (deoxidizer in the blast furnace process), the subsequent reaction is expediently carried out in the presence of elemental iron.
Further digestion possibilities of SiO2 are:
The soda digestion at approx. 1600 ° C in the melting tank:
The hydrothermal digestion at approx. 200 ° C with water in an autoclave:
solar silicon
For the production of solar cells, the raw silicon must be further purified to solar silicon (Sisg). There are different procedures for this. These methods are the most energy-intensive part in the production of solar modules due to the many complex intermediate steps. Therefore, various manufacturing methods such as the UMG method (Upgraded Metallurgical Grade) and the FBR method (Fluidized Bed Reactor) are now being tested and used.
A chlorine-free alternative is the decomposition of monosilane, which decomposes again after a cleaning step on heated surfaces or when passing through fluidized bed reactors.
The polycrystalline silicon (polysilicon) obtained in these ways is suitable for the production of solar modules and has a purity of over 99,99%. In solar technology, as in microelectronics, the semiconducting properties of silicon are exploited.
Only of historical interest is a procedure that was previously used by the company DuPont. It was based on the reduction of tetrachlorosilane with elemental zinc vapor at temperatures of 950 ° C.
Due to technical problems and the large amount of zinc chloride waste, however, this process is no longer used today.
Semiconductor silicon
Monocrystalline semiconductor silicon
For applications in microelectronics, high-purity, monocrystalline silicon (Sieg) is needed. In particular, contaminations with elements which are also suitable as doping elements must be brought to concentrations below certain critical values by crucible pulling or zone melting. The manufacturer Shin-Etsu advertises an "11N" -ref (= 99,999 999 999%) of its ingots.
In crucible pulling (Czochralski process), the solar silicon obtained in the Siemens process is melted in quartz crucibles. A high purity monocrystalline silicon seed crystal is placed in this melt and slowly withdrawn from the melt while spinning to crystallize high purity silicon in monocrystalline form on the crystal leaving almost all contaminants in the melt. The physical background of this purification process is the melting point depression and tendency of substances to crystallize as pure as possible.
Alternatively, during zone melting with the aid of a (ring-shaped) electric induction heater, a molten zone is passed through a silicon rod, whereby a large part of the contaminants dissolves in the melt and migrates with it.
High purity crystalline silicon is currently the most suitable base material for microelectronics; less with regard to its electrical properties than because of the chemical, physical and technical properties of silicon and its compounds (silicon dioxide, silicon nitride, etc.). All common computer chips, memory, transistors etc. use high-purity silicon as starting material. These applications are based on the fact that silicon is a semiconductor. By the targeted incorporation of impurities (doping), such as indium, antimony, arsenic, boron or phosphorus, the electrical properties of silicon can be varied within a wide range. Above all, by means of the thereby generated PN junction effects can be realized a variety of electronic circuits. Because of the increasing importance of electronic circuits, one also speaks of the silicon age. The Silicon Valley name for the high-tech region in California also points to the enormous importance of silicon in the semiconductor and computer industries.
Amorphous silicon can be converted to polycrystalline silicon using excimer lasers. This is of increasing importance for the manufacture of thin-film transistors (TFT) for flat panel displays.
Silicon wafer
Silicon is commercially available both as a fine-grained powder and in larger pieces. High-purity silicon for use in solar modules or in semiconductor components is usually produced in the form of thin disks of single crystals, so-called silicon wafers (see Fig.). Due to the high initial investment and long construction times for the necessary ovens, however, only a few companies worldwide produce raw silicon.
The largest producers of metallurgical silicon are:
- Elkem (N, USA)
- Invensil (F, USA)
- Globe Metallurgical (USA)
- Rima Metal (Br)
There are about 15 other big producers. There are a number of smaller works in the People's Republic of China, making it the biggest producer in the country.
The market for polysilicon and hyperpure silicon has been in transition since the middle of the 2000s. Due to the high demand of the solar industry 2006 came to a silicon shortage.
Physical Properties
Silicon, like the germanium, gallium, phosphorus and antimony adjacent to the Periodic Table, is an elemental semiconductor. The energetic distance between valence band and conduction band according to the band model is 1,107 eV (at room temperature). By doping with suitable doping elements such as boron or arsenic, the conductivity can be increased by a factor 106. In silicon doped in this way, the impurity line caused by impurities and lattice defects is significantly larger than that of the intrinsic line, which is why such materials are referred to as impurity semiconductors. The grid parameter is 543 pm.
Spectrum of the complex refractive index (N = n + ik) of silicon
The complex refractive index, which depends on the wavelength of the light, is shown in the adjacent picture. Information about the band structure can also be read here. The strongly increasing course of the extinction coefficient k shows a direct band transition at 370 nm (E1 = 3,4 eV). Another direct band transition can be observed at ≈ 300 nm (EΓ2 = 4,2 eV). The indirect band transition of silicon (Eg = 1,1 eV) can only be guessed at. The fact that there are additional indirect band transitions can be seen from the wide curve of k for wavelengths> 400 nm.
Like water and a few other substances, silicon has a density anomaly: its density in liquid form (at Tm = 1685 K) is higher by 10-11% than in solid, crystalline form (c-Si) by 300 K.
Chemical properties
In all occurring in nature and in the vast majority of synthetically produced compounds, silicon exclusively forms single bonds. The stability of the Si-O single bond in contrast to the CO double bond is due to its partial double bond character, which results from the overlap of the lone pairs of oxygen with the empty d orbitals of silicon. The double-bond rule, which has long been considered valid, according to which silicon is an element of the 3. However, in the meantime, it has become obsolete since a large number of synthetically produced compounds with Si-Si double bonds are now known. In 2004, the first compound with a formal Si-Si triple bond was structurally characterized.
With the exception of nitric acid-containing hydrofluoric acid (in which hexafluorosilicate is formed), silicon is insoluble in acids because passivation occurs through the formation of a solid silica layer. On the other hand, it dissolves easily in hot caustic alkalis with the formation of hydrogen. Despite its negative normal potential (-0,81 V), it is relatively inert in its compact form, as it covers itself with a protective oxide layer in the air.
Mechanical properties
The mechanical properties of silicon are anisotropic (directional). Depending on the selected crystal orientation, the modulus of elasticity assumes values between 130 GPa and 189 GPa. A general description of the elastic behavior is given in Voigt notation as for all cubic crystals via the three independent elastic constants C11, C12 and C44. The elasticity matrix is for silicon:
The elastic constants have the following values:
The respective elastic moduli can be calculated from the elastic constants for the individual main crystal directions of the silicon (100,110 and 111):
isotope
There are a total of 23 isotopes between 22Si and 45Si of silicon known. Of these, three, the isotopes 28Si, 29Si and 30Si, are stable and naturally occurring. The isotope with the largest share of the natural isotopic composition is 28Si with 92,223%, 29Si has a share of 4,685% and 30Si of 3,092%. The longest-lived unstable isotopes are 32Si, which goes into 153P (phosphorus) with a half-life of 32 years under beta decay, and 31Si, which also decays to 157,36P under beta decay with a half-life of 31 minutes. All other isotopes have only short half-lives of seconds or milliseconds.
28Si is formed in heavy stars towards the end of their development in large quantities (oxygen burning). This is the reason for the high proportion of 28Si in the total silicon (92,23%) and also in the frequency of silicon compared to other elements. Since 2009, attempts have been made to redefine the SI base unit kilogram as a given set of 28Si atoms; these attempts led 2018 to a new definition in November. Also stable are the isotopes 29Si (4,67% share of total silicon) and 30Si (3,1%).
The radioactive isotope 31Si decays rapidly (half-life 157,3 minutes) by beta radiation to stable phosphorus. This circumstance can be used to produce very homogeneously n-doped silicon. For this purpose, silicon is irradiated with neutrons, by neutron capture then arises 31Si and thus 31P. A suitable neutron source for this method is the research neutron source Heinz Maier-Leibnitz. More durable is 32Si with a half-life of 172 years. Traces of this isotope are formed in the earth's atmosphere by spallation of argon by cosmic radiation. 32Si decays to the equally radioactive 32P (half-life 14,3 days), and then further to stable 32S (sulfur). All other isotopes disintegrate within a few seconds.
Safety
Silicon is a combustible powder like many elements. As a powder and granules it is irritating. Compact silicon is harmless.
Hydrogenated, ie superficially hydrogen-covered, porous silicon can be highly explosive under laser irradiation and an increase in oxygen, as researchers at the Technical University of Munich discovered by chance. Blasting in the micrometer range are possible. The detonation speed and detonation energy are higher than for TNT and dynamite.
Use in technology
1947 discovered John Bardeen, Walter Brattain and William Shockley the controllable electrical resistance, the transistor, first on a germanium single crystal. The connection-joyful silicon could be isolated only later in the purity necessary for electronic purposes. 1958 Robert Noyce at Fairchild and Jack S. Kilby at Texas Instruments independently developed the integrated circuit (IC) on a silicon chip. Since about 1970, silicon is the basic material of most semiconductor products and is the base material for many sensors and other micromechanical systems (eg lever arm in an atomic force microscope). Silicon is also the elemental component of most solar cells.
In November 2005 was reported on the first promising results with silicon lasers.
Silicon is used as a high-energy fuel in many explosives.
Since silicon expands upon solidification, while most substances contract, it is added to many cast alloys. For example, cast iron always contains about 2% Si. Of particular importance are aluminum-silicon alloys in which the Si content can be up to 20%. This is the most important variety of all cast aluminum materials.
Connections
Silicon is almost always tetravalent in chemical compounds. Accordingly, the silicon atom in compounds is usually four-coordinate. In addition, there are now a number of compounds in which silicon has a five or sixfold coordination. In addition to the tetravalent silicon and synthetically prepared compounds of divalent silicon (silylenes) are known, but most are very unstable. Of greater importance is only the silicon monoxide, which is used as a material for the compensation of optical lenses. In addition, 2012 has also experimentally detected a three-coordinate compound similar to the one-dimensional structure of graphene, the so-called silicene.
The entire chemistry of silicon is essentially characterized by the high affinity of silicon for oxygen. Silicon is usually the electropositive partner of a chemical compound, although there are also compounds with formally negated silicon. These are mostly silicides where silicon can also form true anions.
![]()
Inversion of binding polarity
Particularly noteworthy is the inversion of the bond polarity of element-hydrogen bonds in the transition from carbon to silicon. Here, the electronegativity difference changes from + 0,45 (carbon-hydrogen) to -0,2, which is why silane compounds have a completely different reactivity than hydrocarbons.
The most important compounds of silicon can be divided into the following classes, of which some representatives are mentioned:
Binary connections
- silicon carbide
- silica
- silicon nitride
- silicides
Silicate
- Zircon and all other silicates and compounds of silicic acid
silicon halides
- Silicon tetrafluoride
- Silicon tetrachloride
- Trichlorosilane (silicochloroform)
Silicon hydrides
- monosilane
- silanes
Organic silicon compounds
- Tetramethylsilane (TMS, NMR standard)
- Methylchlorosilanes such as dichloromethylsilane (building blocks for silicones)
- phenylchlorosilane
- carbosilanes
- Carbosilazane
- carbosiloxanes
Polymeric silicon compounds
- Silicones (silicones, polyorganosiloxanes) are formed by polymerization and belong to the most important industrial plastics.
- Polymeric silicon-oxygen compounds find application in many areas; They serve as lubricants and sealants in the cosmetics and construction industries.
- Polysilanes, -carbosilanes, -carbosilazanes, -carbosiloxanes
Other
To this day, it is often the case that the English word "silicon" (for silicon) is falsely translated or pronounced in popular science articles or in film dubbing as "silicone" ("silicone"). This happened, for example, in the science fiction series Star Trek, the James Bond agent thriller In the face of death or in the animated series The Simpsons. Example: "Is the life form made of carbon or silicone?"
Silicon prices
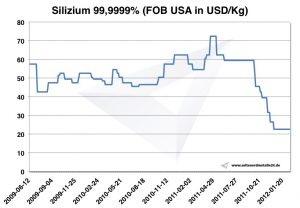
Chart silicon 2009-2012





















