Antimony, Sb, atomic number 51
Antimony price, history, occurrence, extraction and use
antimony
Antimony (from Latin Antimonium, probably from Arabic "al-ithmîd (un)" is a chemical element with the element symbol Sb (from Latin Stibium, (gray) pike gloss) and the atomic number 51 In the periodic table it is in the 5. Period and the 5 main group, or 15, IUPAC group or nitrogen group. In the stable modification, it is a silver-shining and brittle semi-metal.
Name, history
It is also believed that the name goes back to the late Greek anthemon (German bloom). The aim was to describe the stalk-like crystals of antimony sulfide, which appeared as tufts of flowers and looked like a flower. In the 11. Century finds itself the Latin term for the mineral medicine drug antimonium for the internal therapy of illnesses then with Constantinus Africanus.
In the 17. In the 19th century, the name antimony was used to refer to the metal. The Coptic term for the make-up powder antimony sulfide went over the Greek in the Latin stibium. The abbreviation Sb used by the Swedish physician and chemist Jöns Jakob Berzelius ("father of modern chemistry") is still used today as an element symbol.
A late legendary folk etymology immortalized by Samuel Johnson in his dictionary says that the German monk Basilius Valentinus observed that pigs quickly became fat by absorbing antimony. He also tried this on his friars, whereupon they died, however, so that the term "antimoine" (antimony) was coined, from which later "antimony" was created.
A typical locality for solid antimony is the silver mine in the Swedish municipality of Sala in Västmanland. However, metallic antimony was already known to the Chinese and Babylonians. Some of its compounds were already used in the Bronze Age as a supplement to copper to produce bronze (finds of Velem St. Vid in Hungary).
occurrence
Antimony is a rare element. Since it can also be found in nature (ie in elemental form), it is certified by the International Mineralogical Association (IMA) under the system no. 1.CA.05 recognized as a mineral.
Solid antimony (2011) has been detected worldwide at 300 sites. So, inter alia, in several regions of Australia; in the Bolivian departments of La Paz and Potosí; Minas Gerais in Brazil; Black Forest, Fichtelgebirge, Upper Palatinate Forest, Odenwald and Harz in Germany; Seinäjoki in Finland; several regions of France; Lombardy, Piedmont, Sardinia and Trentino Alto Adige in Italy; some regions of Canada; some regions of Austria; Eastern and Western Siberia and the Urals in Russia; besides Västmanland, Dalarna, Gästrikland, Närke, Södermanland, Värmland and Västerbotten in Sweden; in some regions of Slovakia; Bohemia and Moravia in the Czech Republic as well as in many regions of the USA. One of the world's most important deposits of solid antimony and antimony ores is the Murchison greenstone belt in the Murchison Range of South Africa.
So far 264 antimony minerals are known (as of: 2010). Mainly used is the sulphide mineral Stibnit Sb2S3 (Grauspießglanz) with a maximum content of 71,7% Sb. The mineral with the highest Sb content in a chemical compound is the natural antimony arsenic alloy Paradocrasit (max 92%). However, it comes with only three localities, in contrast to Stibnit (around 2500 sites), much less common. Further sources of antimony are the minerals Valentinit Sb2O3 (Weißspießglanz), Breithauptit NiSb (antimony nickel, nickel antimonide), Kermesit Sb2S2O (red spit shine) and Sb2S5 (gold sulfur).
Extraction and presentation
Technically, antimony is extracted from the antimony gloss. One method is based on roasting and reduction with carbon (reductive reduction method):
Another possibility is to carry out the reduction with iron (precipitation method):
Worldwide were at the beginning of the 21. Century between 110.000 and 160.000 tons per year of antimony promoted. Since 1900, the output has increased more than tenfold.
87% of antimony production takes place in China (as of: 2015).
Features
Antimony can occur in three different modifications, with metallic or gray antimony being the most consistent modification.
Under normal conditions, antimony crystallizes trigonal in rhombohedral setup in the space group R3m (no. 166) described according to the Hermann Mauguin symbolism with the lattice parameters a = 431 pm and c = 1127 pm as well as six formula units per unit cell.
By quenching antimony vapor on cold surfaces, amorphous, black and highly reactive antimony is formed, which is converted back into metallic antimony by heating. Electrolytic production produces explosive antimony which, when cracked, becomes explosively glowing and sparks into metallic antimony. However, this form always contains some chlorine and can not be considered a modification. Yellow antimony is also not an independent modification, but a highly polymeric chemical compound with hydrogen.
Physical Properties
Metallic antimony is silver-white, very shiny, flaky-coarsely crystalline. It is easy to crush due to its brittleness. Electrical and thermal conductivity are low.
Chemical properties
With nascent hydrogen, antimony reacts with the unstable antimony hydride SbH3. Air and water do not attack antimony at room temperature. Above the melting point, it burns in air with a bluish-white flame to antimony (III) oxide. It dissolves in hot concentrated mineral acids. With the halogens, it reacts vigorously at room temperature to the corresponding halides.
In compounds, antimony is mainly present in the oxidation states + 3 and + 5. In metal antimonides such as potassium antimonide K3Sb it forms Sb3 ions.
isotope
There are two stable antimony isotopes: 121Sb and 123Sb.
Use and alloys
The majority of antimony produced is processed into alloys and shows the following properties:
It serves to harden lead and tin alloys. In contrast to most other metals, it expands upon cooling of the melt (due to conversion to another modification): the antimony content can be adjusted so that such alloys do not shrink on shrinkage or even expand somewhat; When parts are produced in casting molds, the metal presses during solidification in all corners and angles, so that even complicated shapes and heavily patterned surfaces can be made void-free.
Important alloys:
- Lead-antimony alloys: hard lead, letter metal, bearing metal, accumulator lead, lead sheath for underground cables
- Tin-antimony alloys: Britannia metal, bearing metal
- Production of semiconductors, eg. B. by doping of silicon, for the production of III V compound semiconductors
- Tin-antimony-copper alloys (Babbit metal) for bearing metals
- Tin-antimony-copper-lead alloys for pewter and other articles made of tin
so-called solder or soft solder - Aluminum antimony, gallium antimony, indium antimony for infrared and Hall effect devices
- Non-shrinking antimony alloys for precision casting
Medicine
Antimony (or a preparation derived from antimony ore) was in the 16. and 17. Became a (iatrochemical) "lead medicine", but was - like other Paracelsian drugs - controversial and also prohibited in France between 1615 and 1688.
Tartar stone has long been used as a refractive agent (antimony pill), today it is still sometimes used to study the stomach contents of birds.
Both schistosomiasis and trypanosomes were beginning at the beginning of 19. Century with Brechweinstein (Kaliumantimonyltartrat) fought. Tartar stone was made by storing wine in an antimony cup for one day, and then drinking it. Meanwhile, more effective and more compatible drugs are used.
Antimony supplements are commonly used as less toxic pentavalent forms for drug therapy of leishmaniasis and schistosomiasis, but are no longer the drug of first choice in developed countries. Antimony inhibits the enzyme phosphofructokinase, which is the rate-limiting step in glycolysis.
Additional
- Kindling match
- Part of explosives detonators and leaded ammunition.
- Antimony trisulfide in brake linings of vehicles
Antimony (V) sulphide: - for the production (vulcanization) of red rubber (example: laboratory rubber hoses)
- as a red component of the firing head in matches
- earlier than eye make-up and in ophthalmology ("Augenerweiterer")
- Antimony chromate as a yellow color pigment
antimony oxides - Catalyst for the production of polyester and PET (antimony (III) oxide)
- as white pigment for coloring polystyrene, polyethylene and polypropylene
- Production of white glazes and frits (porcelain)
- Purification of leaded glass
- doped with tin as a transparent-conductive coating ("ATO" antimony-tin-oxides), for example on glasses, for the production of displays or in electrically conductive pigments ("Minatec"), for floor coverings for dissipating electrostatic charges.
- in pigments ("Lazerflair") for the laser marking of plastic parts, because of the strong absorption of infrared radiation usual marking laser (Nd: YAG).
- in camouflage because of the strong infrared absorption.
- as a flame retardant and as a component of flame resistant and flame retardant paints,
- Plastics and textiles for cable sheathing, car seat covers, curtain fabrics, children's clothing and more. Ä.
- Antimony salts as a component of pesticides, stains and fireworks
- Divider for gold: for the precipitation of silver from gold melt
toxicity
Antimony can be fatal on ingestion from 200 to 1200 mg. In toxicology, three antimony forms are known, of which the gaseous antimony hydride (Stiban, SbH3) is the most dangerous form inducing massive hemolysis. Following toxicity, tartar emetic follows with trivalent ("trivalent") antimony, while pentavalent antimony is the least toxic.
The trivalent antimony is taken up in red blood cells within the first two hours after ingestion to 95% and thus enriched mainly in strongly perfused organs. The excretion is predominantly due to binding to glutathione via the bile with a correspondingly high enterohepatic circulation, and only a small part is eliminated via the kidneys. Potassium antimonyl tartrate is excreted to 90% within the first day after ingestion, the remainder being 10% due to a slower elimination kinetics over 16 days.
Antimony, like arsenic, is thought to inhibit the function of the pyruvate dehydrogenase complex and thus lead to a deficiency of the intracellular energy carrier adenosine triphosphate (ATP). This leads to the formation of chelate complexes between the antimony and thiol groups of the corresponding enzymes. In the body, it is toxic in many organs, including the digestive tract, liver, kidneys, heart and central nervous system. The highest concentration reaches antimony in the liver, where it can come to a hepatitis up to the liver failure. At heart there are ECG changes with inversion and reduction of T-wave and prolonged QT interval. Acute renal failure can lead to temporary or permanent hemodialysis.
In addition to supporting measures such as infusion therapy (both to compensate for the loss of fluid through vomiting and to protect the kidneys) and close monitoring of vital signs and the ECG, activated carbon, N-acetylcysteine as a precursor of glutathione, is used for antimicrobial poisoning increased secretion and a chelating agent, eg. B. Dimercaprol.
Results from research indicate that antimony compounds irritate the skin and mucous membranes. These compounds probably dissolve out of plastic and textiles.
Safety instructions and limit values
Of the antimony compounds, the EU has classified antimony fluoride as toxic (T) and the chlorides as corrosive (C) and as dangerous for the environment (N); all other antimony compounds as harmful (Xn) and dangerous for the environment (N). Antimony itself is not listed there, according to the safety data sheet, it is labeled as irritating.
The International Agency for Research on Cancer (IARC) classifies antimony (III) oxide as a possible carcinogenic substance.
In the EU, drinking water has a limit of 5 μg / l. Examinations of fruit juice bottled in PET bottles (for which there are no guidelines) revealed antimony concentrations up to 44,7 μg / L in undiluted juice concentrates.
Antimony was added to 2016 by the EU under Regulation (EC) No 1907 / 2006 (REACH) in the context of substance evaluation in the rolling Community action plan (CoRAP). It will reassess the impact of the substance on human health or the environment and, where appropriate, initiate follow-up. The main reasons for the uptake of antimony were concerns about exposure of workers, high (aggregated) tonnage, high risk ratio (RCR) and widespread use, as well as the potential risk of carcinogenic properties. The revaluation runs since 2018 and is carried out by Germany.
proof
preliminary tests:
Flame coloration: Flame pale blue, little characteristic Phosphorsalzperle: Colorless (disturbed by all elements that produce a colored pearl)
Detection reaction:
Reduction by base metals, for example iron, zinc or tin.
In non-acid solutions, base metals reduce antimony cations Sb (III), Sb (V) and Sb (III) / (V) to metallic antimony:
2 Sb3 + + 3 Fe → 2 Sb + 3 Fe2 +
The substance to be tested for antimony is placed in hydrochloric acid solution and mixed with iron powder. The result is a black, flaky precipitate of metallic antimony in the solution or directly on the iron. The proof of an iron nail is also possible. Here, a black deposit on the nail is a proof of antimony, which has deposited here elementary.
The Marsh sample allows a clear detection of antimony. When the pyrolytically deposited substance (dark glossy mirror) does not dissolve in ammoniacal hydrogen peroxide, arsenic and germanium are excluded as possible alternatives.
The highly sensitive determination of tiny traces of antimony occurs through the hydride technique of atomic spectrometry. In principle, the Marsh sample is coupled with atomic absorption spectrometry. The matrix effects of the sample solution can be very effectively suppressed.
Another method is to treat an aqueous solution containing antimony ions with rhodamine B solution. It forms a colored complex which is extractable with isopropyl ether. However, this evidence is quite unspecific, since gold, cadmium, gallium, thallium, uranium and tungsten ions form colored complexes.
Connections
- Antimony hydrogen, also called monostiban SbH3.
- Toxic gas that is formed from antimony and acids.
- Distiban (Sb2H4)
halogen compounds
- Antimony (V) fluoride (SbF5) forms (according to VSEPR) a square pyramid and hybridizes to sp3d
- Antimony (V) chloride (SbCl5)
- Antimony (III) fluoride (SbF3)
- Antimony (III) chloride (SbCl3)
- Antimony (III) bromide (SbBr3)
- Antimony (III) iodide (SbI3)
oxygen compounds
- Antimony (III) oxide (antimony trioxide, Sb2O3)
- Antimony (III, V) oxide (antimony tetroxide, Sb2O4)
- Antimony (V) oxide (antimony pentaoxide, Sb2O5)
- Antimony acid / antimony trihydroxide (H3SbO3 / Sb (OH) 3)
- Antimony acid / antimony (III) acid, SbOOH or HSbO2
- Antimonic acid (HSb (OH) 6)
sulfur compounds
- Antimony trisulfide, also called antimony gloss (Sb2S3)
Gray-black, shiny metallic stems. Starting material for the production of metallic antimony. Soluble in strong acids. Use for matches, rubbing glasses and camouflage paints (reflection of IR light). - Antimony pentasulfide, formerly known as gold sulfur (Sb2S5)
Other compounds
- Antimony (V) chloride fluoride (SbCl4F) (catalyst for the production of polytetrafluoroethylene ("Teflon"))
- Aluminum Antimonide (AlSb)
- Gallium antimonide (GaSb)
- Indium antimonide (InSb)
Antimony chart 2005-2020
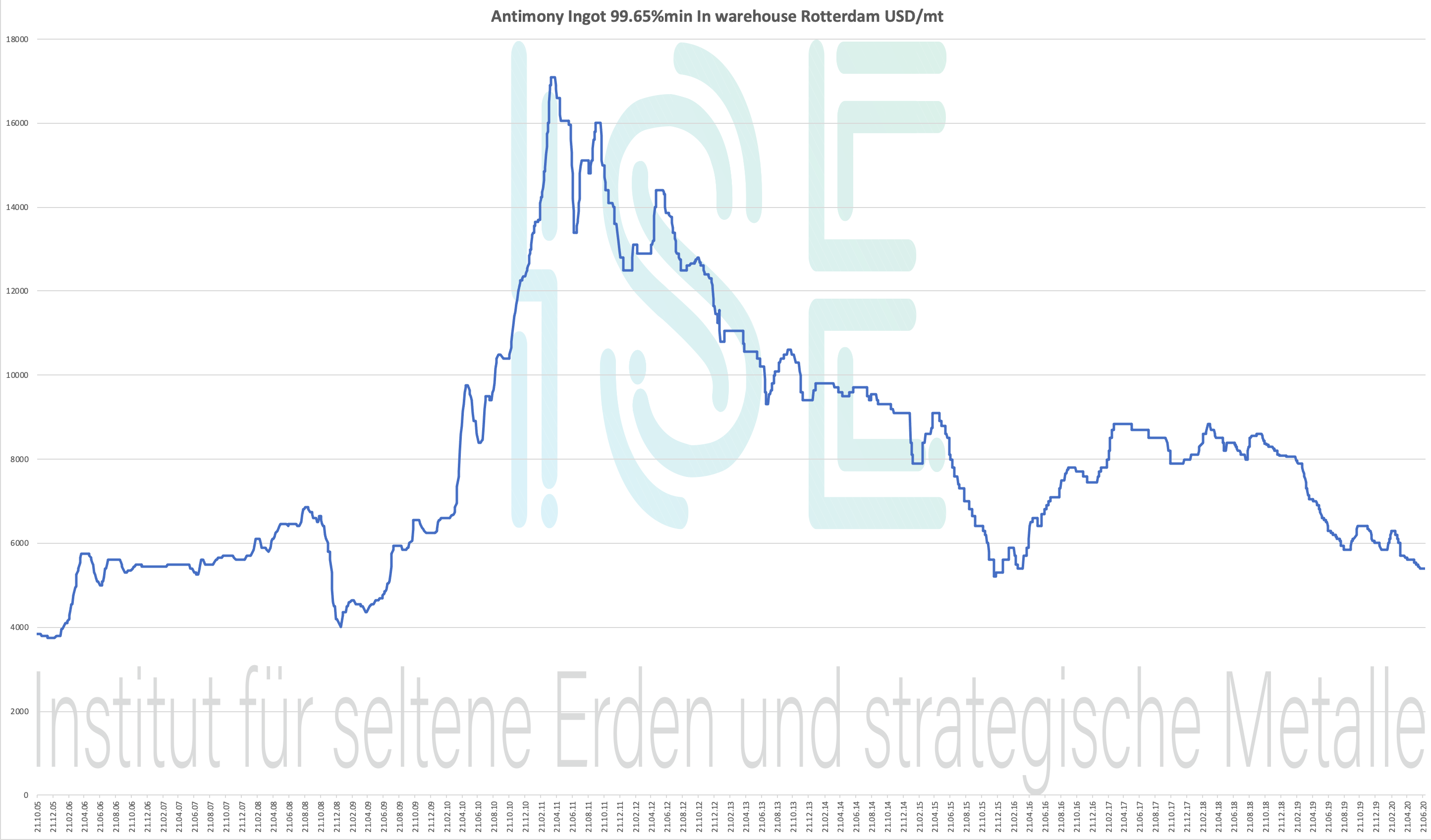
Antimony Ingot 99.65% min In warehouse Rotterdam USD / mt
Price chart 2004-2020 - please click to enlarge
Antimony Price - Below Strategic Metals Price
Historical price data for Antimony Ingot 99,65% warehouse Rotterdam USD / mt



