Chromium, Cr, atomic number 24
Chromium - Chromium extraction, use, prices
General
Chromium (alt. Χρῶμα chrṓma, color ') is a chemical element with the element symbol Cr and the atomic number 24. It belongs to the transition metals, in the periodic table it is in the 6. Subgroup (group 6) or chromium group. The compounds of chromium have many different colors and are often used as pigments in paints and varnishes.
Recovery
The extracted chromite ore is freed from the dead rock. In the second step, an oxidizing digestion takes place at approx. 1200 ° C to give the chromate:
The sodium chromate is extracted with hot water and converted into dichromate with sulfuric acid:
The sodium dichromate crystallizes on cooling as a dihydrate from the solution. A subsequent reduction with carbon gives chromium (III) oxide:
This is followed by the aluminothermic reduction of the chromium (III) oxide to chromium:
Chromium cannot be obtained from oxidic ores by reduction with coal, since this creates chromium carbide. Purer chromium is made by electrodepositioning the Cr3+Ions from sulfuric acid solution shown. Corresponding solutions are prepared by dissolving chromium (III) oxide or ferrochrome in sulfuric acid. However, ferrochrome as a starting material requires a prior separation of the iron.
Extremely pure chromium is produced by further purification steps using the van-Arkel-de-Boer process.
Ferrochrome is produced by reducing chromite in an electric arc furnace at 2800 ° C.
Features
Chromium is a silver-white, corrosion- and tarnish-resistant hard metal that is tough, malleable and malleable in its original state. It is antiferromagnetic with a Néel temperature of 475 K.[11] Chromium dissolves in hydrochloric acid and sulfuric acid after some time with evolution of hydrogen when the protective oxide layer is gone. Common oxidation levels of chrome are +2, +3 and +6, with +3 being the most persistent.
Cr (II) is d4-Configuration unstable. There is hardly any other reducing agent that absorbs oxygen from the air as quickly as Cr (II). But even without air admission, Cr2+-Solutions are only stable for a short time if they are obtained from the purest chrome (e.g. electrolyte chrome).
Cr3+ is the most stable form. This is explained by the crystal field theory, according to which the d3 Configuration is stabilized by a half-filled lower shell.
Cr (VI) as chromate (CrO42−) or dichromate (Cr2O72−) is used as a strong oxidizing agent. It is toxic and carcinogenic. In aqueous solutions, there is a chemical equilibrium between the two ions that is pH-dependent. If you acidify a dilute yellow chromate solution, you give H.+-Ions in addition, according to LeChatelier the equilibrium shifts in the direction of the dichromate, the solution turns orange.

- Safety instructions and biological significance
The role of Cr (III) (Cr3+Ions) in the human body is currently the subject of controversy. There are indications that Cr (III) could have a role in the carbohydrate and fat metabolism of mammals. This information is currently being followed up. Earlier indications that the popular dietary supplement Cr (III) -picolinate has a beneficial effect on body structure could not be confirmed in later studies. A study with hamster cells showed that Cr (III) picolinate is mutagenic and can cause cancer.
The data currently available indicate that it is extremely unlikely to suffer from a chromium deficiency. Even higher doses of Cr (III) trigger a toxic effect with difficulty, since the solubility product of Cr (III) hydroxide is extremely low (6,7 x 10−31). It is therefore presumably very difficult to absorb in the human intestine. In the USA, the recommended intake level of Cr (III) has been reduced from 50–200 µg / day to 35 µg / day in adult men and to 25 µg / day in adult women.
Cr (VI) compounds are extremely toxic. They are mutagenic and damage DNA. They enter the body through the airways and damage the lung tissue. People chronically exposed to such compounds are at an increased risk of developing lung cancer. The poisonous effect increases with the insolubility of the salt. The RoHS directive severely restricts the use of Cr (VI) compounds in Europe.
Usage
Chromium and chromium compounds are used in a wide variety of applications where its durability is used:
- hard chrome plating: galvanic application of a wear protection layer up to 1 mm thick directly on steel, cast iron, copper. Aluminum can also be chrome-plated after an intermediate layer has been applied (hard chrome-plated aluminum cylinders in engine construction).
- Dekorverchromung: Galvanic application of a <1 μm thick Cr layer as a decoration with a corrosion-protecting intermediate layer made of nickel or nickel-copper. Plastic parts are also very often chrome-plated. The achrolyte process is a substitute for decorative chrome plating.
- passivation galvanic zinc layers (chromating)
- alloying element: in corrosion and heat resistant stainless steels and non-ferrous alloys
- Catalyst: to enable or accelerate chemical reactions
- chrome tanning: the main method of making leather
Connections
- Chrome Oxide
- Chromium (III) oxide Cr2O3, is used as enamel paint and for coloring glass (green bottles) (also Cologne bridge green). This is not to be confused with the toxic chrome green.
- chrome yellow
- Lead (II) chromate PbCrO4, used to serve as a brilliant yellow color pigment ("Post yellow"). Due to its toxicity, it is now almost completely replaced by organic color pigments. In analysis it is used for the iodometric determination of lead.
The chrome yellow, which is used as an artist's paint, is a lead sulfate / lead chromate (about 2 PbSO4 · PbCrO4). The pigment was discovered by Louis-Nicolas Vauquelin in 1809 and has been produced commercially in Germany since 1820. Chrome yellow has a high hiding power, its light stability depends on the yellow tone. Chrome yellow is rarely used in oil painting. Vincent van Gogh, however, used chrome yellow a. a. in the famous sunflower paintings executed in oil on canvas. Today, however, these partly suffer from discoloration of the yellow tones.
The art technologist Prof. Christoph Krekel from the Stuttgart Art Academy on the use of the pigment chrome yellow: "The painters have fallen on the chrome yellow, because it is a very brilliant yellow - it has a great color intensity, that is, you could create a much brighter painting with the help of this new yellow hue".
Chrome yellow is also an important color in the counterfeiting analysis of "old" paintings.
- chromium dioxide
- Chromium (IV) oxide CrO2, is a black ferromagnetic powder for the production of magnetic tapes with a better signal-to-noise ratio than conventional iron oxide magnetic tapes because chromium dioxide has a higher coercivity.
- chromic acid
- with the hypothetical structure H2CrO4 exists only in dilute aqueous solution. It's very poisonous. It exists as an anion in some chromates and dichromates.
The anhydride of chromic acid, the very poisonous chromium (VI) oxide CrO3, is known as chromium trioxide.
The orange, very poisonous potassium dichromate K2Cr2O7 is a powerful oxidizing agent: In a sulfuric acid solution, primary alcohols are easily converted into the respective aldehydes, which can be used for the semi-quantitative detection of alcohol in the breath. In the laboratory area it was in the form of chromic acid used for cleaning glassware. Upon contact with chloride ions, however, the volatile, carcinogenic chromyl CrO2Cl2 formed (deduction!). Potassium dichromate is also used as a titrant and as a fixative in industrial dye baths. Potassium dichromate and the also very poisonous ammonium dichromate (NH4)2Cr2O7 are the light-sensitive substance in chrome gelatin layers of early photography (see fine printing process).
- chromite
- (Chrome iron brick, see above) FeCr2O4 is used to make molds for burning bricks.
Chromium prices, charts and historical data
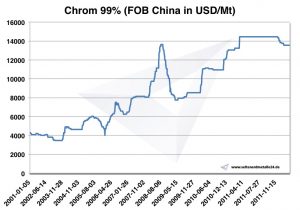
Chart Chrome 2001-2011








 = 3,1 10−4)
= 3,1 10−4)


