Tellurium, Te, atomic number 52
Tellurium price, occurrence, extraction and use
Tellurium [tʰɛluːɐ̯] (Latin tellus "earth") is a rare chemical element with the element symbol Te and the atomic number 52. In the periodic table it is in the sixth main group, or the 16. IUPAC Group, and 5. Period and is one of the Chalkogens. Its frequency is similar to that of gold, with which it also enters into various compounds that occur in nature as minerals. Crystalline tellurium is a silver-white, shiny metallic semi-metal that resembles tin and antimony in appearance. It reacts brittle to mechanical stress and can therefore be easily pulverized. In chemical compounds with non-metals it is close in its behavior sulfur and selenium, in alloys and intermetallic compounds, however, it shows very pronounced (semi) metallic properties.
History
Tellurium was 1782 by the Austrian chemist and mineralogist Franz Joseph Mueller von Reichenstein (1740-1825) in investigations of gold ores from the mine Mariahilf on Mount Faczebaja in Zlatna (German Klein Schlatten, Zalatna) near Sibiu (German Hermannstadt , Transylvania, Romania), which yielded less gold than expected. He had become aware of the ores by Ignaz von Born's (1742-1791) scientific treatise on the dappled Spiesglaskönig in Transylvania. Spiesglaskönig refers to solid antimony, Spies glass is an old name for the mineral Antimonit (Stibnit, Grauss casting Sb2S3). Von Born kept the solid metal in the gold ore for antimony and attributed the low yield to a combination of the gold with antimony. Mueller von Reichenstein contradicted this view and held it first for "sulfurized bismuth". After further investigations, the results of which he published between 1783 and 1785 in a four-part essay, but he also excluded bismuth, since the metal, in contrast to antimony and bismuth, practically did not react with sulfuric acid. He gave the metallic phase the name metallum problematicum (also aurum problematicum or aurum paradoxum). According to current knowledge, there is next to solid tellurium of the minerals Nagyágit (leaves ore, AuPb (Pb, Sb, Bi) Te2-3S6) and Sylvanite (font, (Au, Ag) Te2). Mueller von Reichenstein suggested that metallum problematicum "... perhaps a new as yet unrecognized semi-metal," wanted his findings, however, only by the Swedish mineralogist and chemist Torben Olof Bergman (1735-1784) confirm. In the year 1783, he sent samples of the ore to Bergman for review, but he did not receive definitive answers. Bergman died 1784 and investigations on metallum problematicum have been discontinued for the time being.
It was not until twelve years later, in the year 1797, that Martin Heinrich Klaproth (1743-1817) received samples of the ores from Müller von Reichenstein in Berlin. Klaproth affirmed the conclusions of Müller von Reichenstein's investigations and saw enough evidence for the discovery of a new element. In January 1798 Klaproth praised the merits Müller von Reichenstein in a lecture and attributed to him the discovery of the new element. Since Mueller von Reichenstein had given the element no name, Klaproth decided for the name Tellurium (Latin tellus: "earth"):
To fill this gap in chemical mineralogy, I present here my experiments and experiences with these precious ores, the main result of which is the discovery and validation of a new peculiar metal, to which I add the name Tellurium borrowed from the old Mother Earth. "
The original handpieces of the sample material of the type locality Zlatna, which Klaproth had available, are today in the Museum of Natural History in Berlin.
Independent of Müller von Reichenstein and Klaproth, 1789 discovered the Hungarian chemist and botanist Paul Kitaibel (1757-1817) the tellurium in investigations of gold ore from the mining village Nagybörzsöny (Deutsch-Pilsen) in Hungary. Klaproth mentioned in his published lecture, however, only Müller von Reichenstein, although he had since 1796 by a manuscript Kitaibels also aware of his investigations. In a letter to Kitaibel, Klaproth declared that he had lost the content of the manuscript and that he had not seen any connection with his work in the investigations of the ore Müller von Reichenstein. Finally, Klaproth finally convinced Kitaibel that the discovery of tellurium should be attributed to Mueller von Reichenstein alone, as he made the same observations on the new element several years earlier.
The element symbol "Te" was proposed to 1814 by Jöns Jakob Berzelius (1779-1848) and is still used today. The first structure elucidation of crystalline tellurium with X-ray diffraction was 1924.
occurrence
Tellurium is a rare element; its contribution to the earth's crust is about 0,01 ppm (g / t). With gold, subordinate to silver, copper, lead and bismuth, as well as the platinum metals, it rarely occurs dignified, ie in elemental form in nature.
Solid tellurium belongs as a mineral to the group of elements, more precisely the semi-and non-metals and is in the classification of minerals according to Strunz under the number I / B.03-40 (8 edition) or 1.CC.10 (9. Edition), and led to Dana under the number 1.3.4.2.
Traces to larger amounts of selenium may be contained in solid tellurium (selenium). Although tellurium is a rare element, a relatively large number of minerals are known because tellurium forms its own minerals because it is rarely incorporated into sulfides or selenides or sulfates or selenates; it is too large for these crystal lattices of lighter homologs. Conversely, the two lighter homologs more frequently represent tellurium at its lattice sites in crystal structures of tellurium-containing minerals.
Tellurium shows the highest affinity to gold of all elements and is therefore often found in nature in the form of gold tellurides, minerals with telluride (Te2) or ditelluride anions (Te22-). In addition to gold and other precious metals lead and bismuth form more natural tellurides, often accompanying (paragenesians) to the dignified metals and gold ores.
More rare are minerals with Te4 + cations in the crystal structure, whereby the most important oxide of tellurium, the tellurium dioxide TeO2 occurs in two modifications as orthorhombic tellurite and tetragonal paratellurite in nature. Other tellurium (IV) cations are oxotellurates (IV) (tellurites) containing complex [TeO3] 2 or [TeO4] 4 anions. Minerals containing Te6 + cations in the form of octahedral [TeO6] 6 complex anions are extremely rare, with 21 minerals mostly containing copper and lead. In addition to the mentioned minerals, mixed valence tellurium minerals also exist in nature, including the calcium oxo-tellurate (IV, VI) Carlfriesite CaTe3O8 with a Te4 +: Te6 + ratio of 2: 1. [14] [15] For the minerals with Te4 + and Te6 + - Cations are secondary minerals that have arisen from weathering of tellurium and telluride.
Tellurium-bearing minerals are of no importance for the technical production of tellurium, as they are rare and there are virtually no deposits worth mining. In addition to the type locality Zlatna (Transylvania, Romania), well-known tellurium tellurium-rich minerals include Moctezuma (Mexico), Cripple Creek (Colorado), Kalgoorlie (Australia) and Calaveras (California). So far (as of 2012) 154 tellurium-containing minerals are known, of which, however, five (dilithium, imgreit, kurilite, sztrokayite, protojoseite) have not yet been recognized or discredited as independent minerals by the International Mineralogical Association (IMA).
Extraction and presentation
Tellurium together with selenium is obtained industrially exclusively from byproducts of large-scale electrolytic copper and nickel production. The resulting anode slurries contain water-insoluble noble metal tellurides and -selenides of the general formula M2Ch (M = Cu, Ag, Au, Ch = Se, Te), which at temperatures above 500 ° C. under atmospheric oxygen (O2) with soda (sodium carbonate Na2CO3 ) are reacted. The noble metal cations (M +) are thereby reduced to elemental metals (M), which oxidizes the telluride anions to oxo-tellurates (IV) (TeO32-):
Alternatively, this reaction can also be carried out with nitric acid (sodium nitrate NaNO3) in the absence of air and formation of nitrogen oxides (NO and NO2):
The resulting sodium tellurate (IV) Na2TeO3 is then dissolved in water, where it reacts basicly and forms hydrogentellurate (IV) ions HTeO3-. The separation of the tellurates (IV) from the likewise formed selenates (IV) in the basic solution by neutralization with addition of sulfuric acid (H2SO4), which precipitates in water almost insoluble tellurium dioxide TeO2:
The tellurium dioxide can either be reduced to elemental tellurium in lyes by electrolysis or by chemical means by dissolution in concentrated mineral acids and introduction of sulfur dioxide SO2, the sulfur being formed from the SO2 molecules (or the SOxnumx sulfite ions formed therefrom in the solution). ) and sulphate ions (SO32-) are formed:
The zone melting process is used to obtain high-purity tellurium (> 99,9%).
The global annual production of tellurium increased from 298 tons in 2013 by 53% to 457 tons in year 2017, averaging 382,8 tons per year (t / a). Major producers include China (∅ 220,0 t / a), the USA (∅ 50,0 t / a), Russia (∅ 35,6 t / a), Sweden (∅ 32,4 t / a), Japan (∅ 31,8 t / a) and Canada (∅ 13,0 t / a). An overview of the production quantities of the individual countries is shown in the table. Other industrial nations, such as Germany and Belgium, are likely to produce tellurium, but there are no figures available. [17] The United States Geological Survey (USGS) estimates global reserves of tellurium in 2019 at around 31.000 tonnes.
| Country | 2013 | 2014 | 2015 | 2016 | 2017 | ∅ |
|---|---|---|---|---|---|---|
| China | 150 | 180 | 210 | 279 | 281 | 220,0 |
| USA | 50 | 50 | 50 | 50 | 50 | 50,0 |
| Russia | 31 | 33 | 34 | 40 | 40 | 35,6 |
| Sweden | 24 | 31 | 33 | 39 | 35 | 32,4 |
| Japan | 31 | 32 | 34 | 28 | 34 | 31,8 |
| Canada | 12 | 9 | 9 | 18 | 17 | 13,0 |
| to hum | 298 | 335 | 370 | 454 | 457 | 382,8 |
 |
||||||
modifications
Crystalline tellurium
At standard conditions, only a crystalline modification (Te-I or α-Te) of tellurium is known, which is referred to as crystalline or metallic tellurium. It is isotypic to α-selenium, that is, it has the same crystal structure. In the trigonal crystal system in the space group P3121 (No. 152), tellurium crystallizes with the lattice parameters a = 446 pm and c = 592 pm and three formula units in the unit cell (smallest unit of the crystal structure).
The space group P3121 (No. 152), described according to the Hermann Mauguin symbolism, explains the centering of the unit cell and the existing symmetry elements. P means that the Bravais lattice is primitive. The information on the centering is followed by the existing symmetry elements of the space group: 31 describes a threefold screw axis (multiplication of a particle by rotation by 120 ° and displacement (translation) by 1/3 in the direction of the rotation axis) parallel to the crystallographic c-axis ([001] ), 2 describes a twofold axis of rotation (multiplication by rotation by 180 °) parallel to the three crystallographic a-axes (<100>), 1 the symmetry element of the single axis of symmetry or identity (multiplication by rotation by 360 °, so the particle is formed on itself) in the direction perpendicular to the a-axes and the c-axis (<120>).
The crystal structure contains only one crystallographically distinguishable tellurium atom with the position coordinates x = 0,2636, y = 0 and z = 1 / 3. All other atoms of the crystal structure can be attributed to this atom by the existing symmetry elements of the space group. Since the tellurium atom coincides in its position with the twofold symmetry axis of the space group (P3121 (No. 152)), it is multiplied exclusively by the threefold screw axis (31). This results in helical chains of covalently bound tellurium atoms parallel to the c axis. The tellurium atoms are within the chain 284 pm apart, the bond angle is 103,1 °. The links within the chain are highlighted in red in the illustrations, one chain is shown in blue for clarity, with the dark blue atom on z = 1 / 3, the middle blue on z = 2 / 3 and the light blue on z = 1 or z = 0 is located. Every third atom within the chain is therefore congruent. Each chain is surrounded by six further chains. Van der Waals bonds exist between the chains with Te-Te distances of 349 pm (shown in green dashed lines), which result from falling below the van der Waals radius (2 · 206 pm = 412 pm) of the tellurium atoms , For a single tellurium atom results in a coordination number of 6, more precisely 2 + 4, since 2 atoms come from the same chain and thus have a smaller distance than the other 4 from neighboring chains. As a coordination polyhedron, this results in a distorted octahedron (highlighted in yellow).
Tellurium can also crystallize in the space group P3221 (# 154) instead of P3121 (# 152). The 32 screw axis also duplicates an atom by rotating it around 120 °, but then shifts it by 2 / 3 instead of 1 / 3 in the direction of the axis of rotation. This also creates helical chains that wind in a clockwise direction rather than a counterclockwise direction (in the 31 screw axis) along the c-axis. The crystal structure in the space group P3221 (No. 154) ("left-hand") is thus the mirror image of the structure in the space group P3121 (No. 152) ("legal form"). The appearance of mirror-image crystal forms is called enantiomorphism in crystallography.
The tellurium crystal system is often referred to as hexagonal. The hexagonal and trigonal crystal system is based on the same unit cell, but a hexagonal symmetry would require the presence of a sixfold symmetry axis (6, multiplication of a particle by rotation about 60 °). The crystal structure of tellurium, however, contains only the threefold screw axis (31) and thus belongs without doubt in the lower symmetric trigonal crystal system.
In high-pressure experiments with crystalline tellurium (Te-I or α-tellurium) further modifications were discovered. The specified pressure ranges for the stability of the modifications vary in part in the literature:
- Te-II crystallizes in the monoclinic crystal system in the pressure range from 4 to 6,6 GPa. C2 / m (No. 12) and P21 (No. 4) are mentioned in the literature as possible space groups.
- Te-III crystallizes in the orthorhombic crystal system and is stable in the pressure range above 6,6 GPa. For a orthorhombic modification, a theoretical calculation exists in the space group Imma (No. 74).
- Te-IV crystallizes in the trigonal crystal system in the space group R3m (No. 166) and corresponds to the structure of the β-polonium. It is stable in the pressure range from 10,6 to 27 GPa. The distances of the tellurium atoms within the chains and to adjacent chains in this modification are the same and each amount to 300 pm, which results in the higher symmetry compared to α-Te.
- Te-V is stable above 27 GPa. For this modification, a cubic-body centered lattice (space group Im3m (# 229)) is assumed.
Amorphous tellurium
The unstable amorphous modification is a brown powder and can be prepared from telluric acid (H2TeO3) by reaction with sulfurous acid (H2SO3) or sulfite ions (SO32-). The sulfite ions are thereby oxidized to sulfate ions (SO42-) while the Te4 + cations are reduced to elemental tellurium:
Amorphous tellurium slowly converts to crystalline modification under standard conditions.
Physical Properties
Crystalline tellurium is an intrinsic direct semiconductor with a band gap of 0,334 eV. As with all semiconductors, the electrical conductivity can be increased by increasing the temperature or exposure, but this only leads to a slight increase in tellurium. The electrical conductivity and thermal conductivity behave directionally dependent on tellurium, that is anisotropic. Crystalline tellurium is a soft (Mohs 2,25) and brittle material that is easy to process into powder. By increasing pressure Tellurium transforms into further crystalline modifications. Above 450 ° C, tellurium passes into a red melt, and at temperatures above 990 ° C, tellurium is present as a yellow diamagnetic gas of Te2 molecules. At temperatures above 2000 ° C, Te2 molecules break down into single atoms.
Chemical properties
Crystalline tellurium is insoluble in water and poorly soluble in the mineral acids hydrochloric acid and sulfuric acid and in alkalis. On the other hand, it is very soluble in nitric acid, as it is a very strong oxidizing agent and oxidizes elemental tellurium to tellurates with the stable oxidation state + IV. Tellurium melts attack copper, iron and stainless steel.
In compounds with non-metals, tellurium behaves like the lighter group member selenium. In the air it burns in a green-lined, blue flame to tellurium dioxide TeO2:
Tellurium spontaneously reacts with halogens to form tellurium halides. Remarkably, in contrast to the lighter homologues selenium and sulfur, tellurium also forms thermodynamically stable iodides, including tellurium iodide TeI with the oxidation state + I. With base metals such as zinc, it reacts violently to the corresponding tellurides.
isotope
Tellurium is known for mass-numbered isotopes between 105 and 142. Natural tellurium is a mixed element consisting of eight isotopes, of which five (122Te, 123Te, 124Te, 125Te, 126Te) are stable. The isotope 123Te should theoretically decay to 123Sb under electron capture. However, this decay has not yet been observed; the lower limit for its half-life is 9,2 · 1016 years (92 quadrillion years). The isotope 120Te transitions directly into 120Sn via the double electron capture. The isotopes 128Te and 130Te convert to 128Xe and 130Xe, respectively, by emission of beta radiation (double beta decay).
The major contributor to natural tellurium is about one third of the isotope 130Te with a half-life of 7,9 · 1020 years, followed by the isotope 128Te. The average atomic mass of the natural tellurium isotopes is therefore 127,60, which is larger than that of the pure element iodine with 126,90 following in the periodic table. 128Te is considered the isotope with the slowest decay of all non-stable isotopes of all elements. The extremely slow decay with a half-life of 7,2 · 1024 years (7 quadrillion years, ie in 1 kilograms one atom decays every 18 months) could only be determined by detecting the decay product (128Xe) in very old samples of natural tellurium.
Of the other isotopes, the core isomer 121mTe has the longest half-life with 154 days. Also for the isotopes 127Te and 129Te, the half-lives of the isomers are above those of the ground state. The most commonly used tracer is the isotope 127Te, followed by 121Te. The isotopes 127Te and 129Te also occur as fission products in nuclear fission in nuclear reactors.
Usage
Tellurium is a technically less important element because it is expensive to manufacture and other elements or compounds are often equivalent in use. In 2016, elementary, polycrystalline and doped tellurium thermoelectric behavior with a high figure of merit in the range between room temperature and 400 ° C was demonstrated. Elemental tellurium is used in the metal industry as an additive (<1%) for steel, cast iron, copper and lead alloys and in stainless steels. It promotes corrosion resistance and improves mechanical properties and machinability. Pure tellurium has so far been used only rarely as a semiconductor; tellurium is mostly used in II-VI compound semiconductors. Cadmium telluride CdTe is z. B. used in photodiodes and thin-film solar cells to generate electricity from light.
Bismuth telluride Bi2Te3 is used in thermocouples to generate electricity in thermoelectric generators (eg in radionuclide batteries) or in Peltier elements for cooling.
Combinations of germanium GeTe and antimony telluride Sb2Te3 are used in phase change materials as part of optical storage disks (eg CD-RW) or in novel storage materials such as phase change random access memory.
Due to their high refractive indices, TeO2 tellurium dioxide glasses are used instead of SiO2 silica glass in optical waveguides.
In microbiology, colorless potassium tellurate (IV) K2TeO3 mixed agar is used as a selective nutrient medium for the detection of staphylococci and Corynebacterium diphtheriae. The bacterial colonies appear as small black spheres, as they reduce the Te4 + cations to elemental tellurium and store them in their cells.
Tellurium (or potassium tellurate) was first used medicinally by 1890 for the treatment of nocturnal sweating in patients with tuberculosis.
Furthermore, small amounts of tellurium are used for the vulcanization of rubber, in detonators and for the dyeing of glass and ceramics. The salts of tellurium are partly used to produce a grass green color in fireworks.
Safety instructions and toxicity
In its soluble form, tellurium is a poisonous element for the human organism and was therefore classified as poisonous in the past. However, since elemental tellurium is very poorly soluble in water and the body's own acids, it has been downgraded to harmful. Studies by the Netherlands Organization for Applied Scientific Research (TNO) showed that the LD50 (oral) value for rats is> 5000 mg / kg. The value of 83 mg / kg given in many safety data sheets from the book Toxicometric Parameters of Industrial Toxic Chemicals under single Exposure by NF Ismerow, which dates from 1982, [26] only applies to easily soluble tellurium compounds. In spite of this, various manufacturers continue to use the old LD50 value for elemental tellurium (powder) and the classification toxic in connection with the H-phrase 301 ("Toxic if swallowed").
Tellurium is not as toxic as selenium. This is analogous to the neighboring elements of the 5. Main group, where the antimony is also less toxic than the arsenic. If tellurium, especially in the form of readily soluble tellurium compounds such as alkali metal tellurates (eg Na2TeO3) by ingestion (peroral) in the body, forms by reduction toxic dimethyltelluride (Me2Te: H3C-Te-CH3), which causes damage to blood, liver Can cause heart and kidney. Since readily soluble tellurium compounds release far more tellurium, they are also classified as more dangerous. Tellurium poisoning is manifested in an intense garlic odor of breathable air, which is caused by dimethyltelluride, first by Christian Gottlob Gmelin, 1824 (for his first-ever studies on the effect of tellurium on living things). This will take several weeks to develop and develop even at very low levels, which do not cause serious poisoning. This garlic smell, unlike real garlic, can not be removed by brushing your teeth. It also settles in a room and only moves away after several hours. It is also slowly excreted through the skin.
Tellurst dusts can ignite spontaneously in the air and, when finely distributed in a corresponding concentration, can also react explosively, forming in each case tellurium dioxide TeO2. Like other metal dusts, tellurium powder can also react explosively with interhalogen compounds such as bromine pentafluoride BrF5. A maximum workplace concentration (MAK) for tellurium has not been established.
proof
Elemental tellurium can be detected in hot concentrated sulfuric acid (H2SO4) by oxidation of tellurium to form the red Te42 + cation (tetratelluric dication). Part of the sulfuric acid is reduced in the reaction to sulfuric acid (H2SO3), which decomposes due to the high temperatures in water (H2O) and its anhydride sulfur dioxide (SO2), which escapes as gas:
The color of the square-planar Te42 + cation is due to six delocalized π electrons that absorb part of the visible light. The remaining, unabsorbed wavelengths of light give the complementary color red.
Tellurate and tellurite can be specified by means of polarography, ie selectively determined side by side. While the level of tellurate is -1,66 V, that of tellurite appears at -1,22 V (vs. SCE, 0,1 M caustic soda). Both tellurium species are reduced in one step to telluride. Traces of 0,03% tellurate or 0,003% tellurite are detectable in this way. Significantly stronger are the methods of atomic spectroscopy. While the flame AAS achieves a detection limit of 20 μg / l, this value is much lower for the graphite tube AAS (0,2 μg / l) and the hydride technique (0,02 μg / l).
tellurium
In compounds, tellurium occurs most frequently in the oxidation states -II (tellurides) and + IV (tetrahalides, tellurium dioxide and tellurates (IV), outdated tellurites). More rare are the oxidation states + VI (tellurates (VI)) and + II (dihalides) as well as -I (ditellurides) and + I (monohalides, only known as TeI).
Hydrogen compounds
Telluric Hydrogen H2Te is a colorless, highly toxic gas produced by the reaction of tellurides (MxTey) with strong acids, such as hydrochloric acid HCl. From the elements (hydrogen and tellurium), the compound can be represented as a strongly endothermic compound only at temperatures above 650 ° C. Dissolved in water (telluric acid), it reacts acidic, the acidity corresponds approximately to the phosphoric acid. In the air, the aqueous solution immediately decomposes into water and elemental tellurium.
oxygen compounds
Tellurium dioxide (tellurium (IV) oxide) TeO2 is a colorless crystalline solid and the most important oxide of tellurium. It results from the combustion of elemental tellurium with air. It is the anhydride of the weakly amphoteric and unstable telluric acid H2TeO3. Tellurium dioxide exists in an orthorhombic (tellurite) and a tetragonal (paratellurit) modification, which also occur in nature as minerals.
Tellurium trioxide (tellurium (VI) oxide) TeO3 is a yellow, trigonal / rhombohedral crystallizing solid and the anhydride of orthotelluric acid H6TeO6. It results from the drainage of orthotelluric acid by a strong increase in temperature. The yellow color is due to electron transfer of the oxygen to the tellurium ("charge transfer").
Tellurium monoxide (tellurium (II) oxide) TeO is another, but under standard conditions unstable oxide of tellurium. It is described as a black amorphous solid and reacts in moist air with oxygen to form the more stable tellurium dioxide TeO2.
Dicellular pentoxide (tellurium (IV) tellurium (VI) oxide) is a mixed tellurium oxide with Te4 + and Te6 + cations. It is in addition to tellurium trioxide another product in the thermal decomposition of orthotelluric acid and crystallized in the monoclinic crystal system.
Tellurates are the salts of the orthotelluric acid H6TeO6 and metatelluric acid H2TeO4 with the anions [TeO6] 6- or [TeO4] 2-. The salts of the telluric acid H2TeO3 with the anion [TeO3] 2- are called tellurates (IV) (obsolete tellurites).
halogen compounds
Tetrahalides TeX4 with tellurium in the oxidation state + IV are the most common tellurium halides. These are known with all halogens (fluorine, chlorine, bromine and iodine). All compounds are crystalline solids.
Dihalides TeX2 with tellurium in the oxidation state + II are known only with chlorine, bromine and iodine, they exist only in the gas phase.
Monohalides TeX exist of tellurium only with iodine as tellurium iodide TeI. It is the only known thermodynamically stable mono-iodide of chalcogens and a dark crystalline solid. Tellurium in this compound has the unusual oxidation state + I.
Subhalides contain Te with an oxidation state that is less than + I. Stable representatives are Te2I, Te2Br and Te3Cl2.
Hexahalides TeX6 with tellurium in the oxidation state + VI are known only as tellurium hexafluoride TeF6 or tellurium pentafluoride chloride TeF5Cl. Both are colorless gases. Tellurium hexafluoride is the most reactive chalcogen hexafluoride (besides sulfur hexafluoride SF6 and selenium hexafluoride SeF6) and is the only one to be hydrolyzed in water.
Furthermore, tellurium in the oxidation state + IV in aqueous solution also complex compounds [TeX6] 2- (X = F-, Cl-, Br-, I-) with all halide ions. With the exception of the hexafluoro complex, all others are perfectly octahedral in structure and can also be precipitated as salts from solution (for example, yellow ammonium hexachloridotellurate (IV) (NH4) 2 [TeCl6], reddish brown ammonium hexabromidotellurate (IV) (NH4) 2 [TeBr6] or black cesium hexaiodidotellurate (IV) Cs2 [TeI6]).
Organotellurium connections
Tellurium forms a series of organometallic compounds. However, these are very unstable and are little used in organic synthesis. As pure tellurium organyl compounds of the form R2Te, R2Te2, R4Te and R6Te (R are each alkyl, aryl) are known.
In addition, diorganotellur dihalides R2TeX2 (R = alkyl, aryl; X = F, Cl, Br, I) and triorganotellurhalides R3TeX (R = alkyl, aryl, X = F, Cl, Br, I) are also known.
Tellurpolykationen
Polycation Te72+ in Te7[Be2Cl6]
By careful oxidation of tellurium, in addition to the already mentioned Te42 +, numerous tellurium polycations Tenx + can be prepared and crystallized with a suitable counterion. The counterion must be a weak Lewis base since the tellurium polycations are relatively strong Lewis acids. Suitable oxidizing agents are often halides of the transition metals, which at temperatures of typically 200 ° C give directly the desired compound:
Frequently, crystallization is successful under the conditions of chemical transport, but sometimes anhydrous solvents such as stannic chloride or silicon tetrabromide must be used. In certain cases, molten salts are also suitable reaction media. If the metal halide is not a suitable oxidizing agent, as is generally the case with halides of the main group elements, the corresponding tellurium tetrahalides can be used as oxidizing agents:
By varying the counterion and the reaction medium, a wide variety of polycations could be represented; Mixed selenium-tellurium polycations are also accessible by appropriate choice of the reactants of the synthesis. In addition to the shown chain or band-shaped polycations, there are also isolated polycations such as Te62 +, Te64 + and Te84 +.
Tellurium price
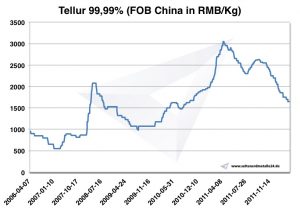
Chart Tellurium 2006-2011
Tellurium prices -> prices for strategic metals










![{\ mathrm {8 \ Te \ + \ 2 \ UBr_ {5} \ longrightarrow \ Te_ {8} [U_ {2} Br _ {{10}}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/766123b2cbd2af42e846172c00731419fb95bc1b)
![{\ mathrm {13 \ Te \ + \ TeCl_ {4} \ + \ 4 \ BeCl_ {2} \ longrightarrow \ 2 \ Te_ {7} [Be_ {2} Cl_ {6}]}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c1d391160b6e1fdb357b4a11ea7abd720a1cb0ba)
