Magnesium, Mg, atomic number 12
Magnesium price, history, occurrence, extraction and use
Magnesium is a chemical element with the element symbol Mg (Alchemy: ⚩) and the atomic number 12. In the periodic table of the elements it is in the second main group or the 2. IUPAC group and thus belongs to the alkaline earth metals.
Magnesium is one of the ten most common elements of the earth's crust. It occurs in numerous minerals as well as in the leaf green of the plants.
History
The origin of the element designation is shown differently in the literature:
- of ancient Greek μαγνησία λίθος meaning "magnetic stone",
- Magnisia, an area in eastern Greece,
- from Magnesia, a city in Asia Minor in the territory of today's Turkey.
However, all the derivations given here seem etymologically to stem from the magnets or their eponymous Heros Magnes.
Magnesium compounds were known and used for centuries before the production of elemental magnesium. Magnesia alba designated magnesium carbonate, while magnesia was the common name for magnesium oxide.
The Scottish physicist and chemist Joseph Black was the first of the magnesium compounds in the 18. Century systematically examined. 1755 in his work De humore acido cibis orto et magnesia alba he recognized the difference between lime (calcium carbonate) and magnesia alba (magnesium carbonate), which were often confused at that time. He took Magnesia alba as the carbonate of a new element. Because of this, Black is often called the discoverer of magnesium, though he never represented elemental magnesium.
1808 won Sir Humphry Davy Magnesium magnesium hydride moistened by electrolysis with the aid of a Volta ash column - not in pure form, but as an amalgam, since he worked with a cathode of mercury. So he showed that Magnesia is the oxide of a new metal, which he first called Magnium.
By heating dry magnesium chloride with potassium as a reducing agent, 1828 succeeded in producing small amounts of pure magnesium for French chemist Antoine Bussy. 1833 was the first to make magnesium by electrolysis of molten magnesium chloride. Based on these experiments, the German chemist Robert Wilhelm Bunsen worked in the 1840 and 1850 years on processes for the production of magnesium by electrolysis of molten salts using the Bunsen element he developed. 1852 developed an electrolysis cell to produce larger quantities of magnesium from molten anhydrous magnesium chloride. This process is still preferred today for the recovery of magnesium.
The technical production of magnesium began 1857 in France after a procedure of Henri Etienne Sainte-Claire Deville and H. Caron. In the so-called Deville-Caron process, a mixture of anhydrous magnesium chloride and calcium fluoride is reduced with sodium. In England, Johnson Matthey began manufacturing 1860 using a similar process. However, due to manufacturing difficulties, these early ventures remained uneconomic.
occurrence
Magnesium does not exist in nature because of its reactivity in elemental form. As a mineral, it occurs predominantly in the form of carbonates, silicates, chlorides and sulfates. In the form of dolomite, a magnesium mineral is even mountain-forming, such. B. in the Dolomites.
The most important minerals are dolomite CaMg (CO3) 2, magnesite (bitterspar) MgCO3, olivine (Mg, Fe) 2 [SiO4], enstatite MgSiO3 and kieserite MgSO4 · H2O.
Other minerals are:
- Serpentine Mg3 [Si2O5] (OH) 4
- Talc Mg3 [Si4O10] (OH) 2
- Sepiolite Mg4 [Si6O15] (OH) 2
- Schönit K2Mg (SOX NUMBER) 4 · 2 H6O
- Carnallite KMgCl3 · 6 H2O
- Spinel MgAl2O4
When dissolved in water, it causes water hardness together with the calcium. In seawater it is contained more than 1 kg / m³.
Extraction and presentation
The extraction of magnesium occurs mainly via two ways:
- By Melt-Flow Electrolysis of Molten Magnesium Chloride in Downs Cells: Downs cells consist of large iron troughs that are heated from below. As anodes serve from above embedded graphite rods, which are surrounded at the tips of an annular cathode. The metallic magnesium accumulates on the molten salt and is skimmed off. The resulting chlorine gas collects in the upper part of the cell and is reused to produce magnesium chloride from magnesium oxide. To lower the melting point of the magnesium chloride, calcium chloride and sodium chloride are added to the molten salt.
- By thermal reduction of magnesium oxide (Pidgeon process): In a container made of chromium-nickel steel is burnt dolomite, barite and a reducing agent such as ferrosilicon, filled. It is then evacuated (pumped out of the gas) and heated to 1160 ° C. The vaporous magnesium condenses on the water-cooled head pipe outside the furnace. The recovered magnesium is further purified by vacuum distillation.
The Pidgeon process is today the most significant manufacturing process and is mainly used in China.
88% of the world's magnesium production takes place in China, where 2015 produced approximately 800.000 t of magnesium metal. This is followed by only a few percent each market share Russia, Israel and Kazakhstan.
The production of 1 kg of magnesium by the Pidgeon process produces greenhouse gases with a CO2 equivalent of about 31 kg (for comparison: for 1 kg of steel, CO0,5 equivalents are formed between 2 and 2 kg).
Although magnesium is contained in more than 60 minerals, only dolomite, magnesite, brucite, carnallite, talc and olivine are of commercial importance.
The Mg2 + cation is the second most abundant cation in seawater, making seawater and sea salt attractive commercial sources of magnesium. To extract it, calcium hydroxide is added to seawater to form a precipitate of magnesium hydroxide.
Magnesium hydroxide (brucite) is insoluble in water and can be filtered off and reacted with hydrochloric acid to give concentrated magnesium chloride.
Electrolysis produces magnesium chloride from magnesium chloride.
Features
The solid, silvery shiny magnesium alloy is about one third lighter than aluminum. Pure magnesium has a low strength and hardness. Its modulus is about 45 GPa. In air, magnesium is coated with an oxide layer, which, in contrast to aluminum, is not completely opaque. The reason for this is that the magnesium oxide has a lower molar volume than magnesium itself (MgO: 10,96 cm3 / mol, Mg: 13,96 cm3 / mol); s. Pilling-Bedworth ratio.
Thin strips or foils are easily ignited. It burns in air with a bright white flame to magnesium oxide MgO and little magnesium nitride Mg3N2. Freshly made magnesium powder can heat up in the air until it ignites spontaneously. Dangerous reactions are to be expected at higher temperatures, that is especially with molten liquid. Also in many oxides such as carbon monoxide, nitrogen oxide and sulfur dioxide burns magnesium.
Magnesium reacts with water to form hydrogen:
Reaction of magnesium with water
This forms a poorly soluble coating of magnesium hydroxide, which largely stops the reaction (passivation). Even weak acids, such as ammonium salts, are sufficient to dissolve the hydroxide layer, as they convert the hydroxide ions to water and form soluble salts. Without passivation, the exothermic reaction proceeds violently; the finer the magnesium dust, the more violent. With air, the released hydrogen easily forms an explosive mixture (oxyhydrogen gas).
Magnesium reacts exothermally with carbon dioxide to form magnesium oxide and carbon:
Reaction of magnesium with carbon dioxide
Therefore, carbon dioxide does not extinguish magnesium fires, but fuels them.
It is relatively resistant to hydrofluoric acid and bases, in contrast to aluminum. The reason for this is the low solubility of the magnesium fluoride (MgF2), which prevents further formation of Mg (OH) 3 ions.
isotope
There are a total of 21 isotopes between 19Mg and 40Mg of magnesium known. Of these, three, the isotopes 24Mg, 25Mg and 26Mg are stable and occur in nature. The isotope with the larger proportion of the natural isotopic composition is 24Mg with 78,99%, 25Mg has a share of 10,0% and 26Mg of 11,01%. The longest-lived unstable isotopes are 28Mg, which turns into 20,915Al with a half-life of 28 hours under beta decay, and 27Mg, which also decays to 9,435Al with a half-life of 27 minutes under beta decay. All other isotopes have only short half-lives of seconds or milliseconds.
Usage
Metallic magnesium
Magnesium powder and wire is used in incendiary devices, bombs and light ammunition, formerly also as flash powder. Often, magnesium rods act as sacrificial anodes, protecting parts of more noble metals from corrosion.
In metallurgy, magnesium finds versatile use,
- as a reducing agent in the Kroll process for the recovery of titanium,
- as a reducing agent for the recovery of uranium, copper, nickel, chromium and zirconium,
- as a constituent of aluminum alloys of the groups AlSiMg and AlMg,
- as magnesium granules for the desulphurisation of iron and steel,
- as aggregate for nodular cast iron,
- as the basis of a group of standardized lightweight alloys for the construction of aircraft and motor vehicles (these melts require a covering layer of molten magnesium chloride for protection against air ingress and oxidation, see melt treatment),
- as fuel for torches that burn under water.
In organic chemistry, it is used to make Grignard compounds.
Because magnesium ignites very easily, it is also used as a very robust lighter. These magnesium blocks, marketed as Fire Starter Kits, have on one side a rod whose abrasion spontaneously ignites in the air, like the flint of a lighter. The procedure is very similar to the Stone Age method of burning flint and tinder, with magnesium playing the role of tinder. First, chips are scraped off the magnesium block with a knife and placed on or under the actual fuel. Then, by scraping on the back "flint" (eg with the back of the knife) sparks are generated as close as possible to the magnesium turnings in order to ignite them.
magnesium alloys
The most important feature of magnesium alloys, which has made them more important than aluminum and its alloys, is the lightweight construction they allow. With a density of around 1,75 g / cm³, the difference to aluminum lightweight construction with a density of 2,75 g / cm³ is clear. In addition, the melting range between 430 and 630 ° C, ie energy-saving lower. However, the mechanical properties such as tensile strength and hardness are significantly lower than with aluminum alloys. The low density made magnesium interesting for mobile applications early on. The first major application took place before the First World War in the construction of the scaffolding for the rigid Zeppelin airships. In automobiles, magnesium alloys were used to make body parts and rims for all kinds of mobile applications. 1930 has increasingly used magnesium alloys in aircraft construction because of its weight savings, more energy-efficient flights and higher payloads. All this led to a rapid expansion of magnesium production in Germany (Elektron from the chemical factory Griesheim) and after 1940 also in the US. "Elektron" became a trademarked name for the first magnesium alloys immediately after the start of production.
Other uses for magnesium casting offered themselves in the course of technical development, partly war-related, partly constructive forward-looking and at the same time optimizing the alloys. As magnesium-based materials, the alloys Mg-Al, Mg-Mn, Mg-Si, Mg-Zn and finally Mg-Al-Zn alloys have been developed.
The gear case of the VW Beetle was cast in millions of layers of a Mg-Si alloy. Today, magnesium alloys are not only used from the viewpoint of weight saving, but they are also characterized by high attenuation. This leads to a reduction of vibration and noise emission when subjected to vibration. Also for this reason, magnesium alloys have become interesting materials in engine construction, such as in the automotive industry. So not only parts of the engine are made of magnesium alloy, but increasingly also for the casting of engine blocks, the hybrid method / hybrid casting applied, for the first time in mass production in the Alfa Romeo 156, later also at BMW (see also BMW N52).
In die casting (see also under molding) can be many, even large-scale, thin-walled components close to the final dimensions and produce without costly reworking, such. As rims, profiles, housings, doors, hoods, bootlid, handbrake lever and other. Not only in the automotive industry, but also in mechanical engineering is constructed with parts of Mg-Al-Zn alloys.
The efforts for lightweight construction already led to the end of the 20. To magnesium-lithium alloys, even lighter alloys of magnesium with the addition of lithium.
Magnesium materials in medicine
Recent research promises a high developmental potential of magnesium materials as resorbable implant material (eg as a stent) for the human body. Magnesium materials must be protected against contact corrosion in the application. The corrosion resistance against normal atmospheric influences, however, is good. The contact corrosion behavior would be a decisive advantage when used as temporary limited implant material, as it would dissolve safely after a certain time. This eliminated the risks and costs of an implant removal surgery.
Fertilizer
In the liming of arable and grassland areas, magnesium in the form of magnesium oxide or magnesium carbonate is used to compensate for the magnesium depletion of the soil by the plants again. Furthermore, the soil pH is increased and the availability of other nutrients is improved. Here, the magnesium compound is usually applied together with lime as magnesium and calcium-containing complex fertilizer. The naturally occurring as Bobierrit magnesium phosphate Mg3 (PO4) 2 (trimagnesium phosphate) and magnesium nitrate are used as complex fertilizer.
physiology
Magnesium is one of the essential substances and is therefore indispensable for all organisms. In the leaf-green of the plants, the chlorophyll, magnesium is contained to about 2%. There it forms the central atom of chlorophyll. In magnesium deficiency plants hibernate as well as in lack of light. Also, the human body magnesium must be supplied daily in sufficient quantities to prevent magnesium deficiency.
The body of an adult contains about 20 g of magnesium (for comparison: 1000 g of calcium). In blood plasma, magnesium is bound to 40% of proteins; the normal serum level is 0,8-1,1 mmol / l. Magnesium is involved in some 300 enzyme reactions as an enzyme constituent or coenzyme. In addition, free Mg ions influence the potential of the cell membranes and act as second messengers in the immune system. They stabilize the resting potential of excitable muscle and nerve cells and the cells of the autonomic nervous system. Magnesium deficiency causes restlessness, nervousness, irritability, lack of concentration, tiredness, general feeling of weakness, headache, cardiac arrhythmia and muscle spasms. It can also come to a heart attack. In the area of metabolism and psyche, magnesium deficiency is thought to exacerbate depression and schizophrenic psychosis. An excess of magnesium in the blood can occur as a result of excessive intake and renal dysfunction and leads to disturbances in the nervous system and the heart.
Magnesium resorption occurs first in the upper small intestine, but also in the rest of the digestive tract. It is excreted by the kidneys and is present in varying amounts in all foods as well as in drinking water. The required daily dose of about 300 mg is usually achieved through a well-balanced diet. An increased need can be covered by supplements or medicines. Slight magnesium deficiency is possible due to serious illness, pregnancy or competitive sports. Severe deficiencies occur in renal dysfunction, prolonged diarrhea, chronic intestinal inflammation, poorly controlled diabetes mellitus, corticosteroids, certain diuretics or alcoholism with malnutrition.
Magnesium salts such as citrate, gluconate, aspartate and aspartate hydrochloride are approved as drugs in Germany, in daily doses of 100 to 400 mg against deficiency states and neuromuscular disorders such as muscle spasms, migraine or pregnancy complications. Side effects include gastrointestinal discomfort and diarrhea, with overdose also fatigue and pulse slowing down. Contraindications include renal dysfunction and certain cardiac arrhythmias.
For oral intake of magnesium supplements (tablets, chewable or lozenges, granules for dissolution in liquid) on the one hand the dosage is important. Several studies have concluded that when taking 120 mg approximately 35% is absorbed, but when taking a complete daily dose of 360 mg only about 18% is absorbed. For absorption in the body, the form of the compounds used today in medicines is irrelevant, since they are pharmacologically as well as biologically and clinically equivalent; Organic salts such as magnesium aspartate or magnesium citrate are only absorbed faster by the body than inorganic compounds. On the other hand, the additional magnesium remains beneficial in the body only if enough binding molecules in the body are available; This is done by biochemical adjustments only after a prolonged increase in magnesium supply or intake over at least four weeks. Magnesium sulfate ("Epsom salt") was formerly used as a laxative. Magnesium salts are used in alternative medicine.
Food
Magnesium serves as a cofactor for 300 various proteins, especially ATP- and nucleic acid-binding enzymes. The recommended daily intake of magnesium in humans, depending on age and gender, is between 24 and 400 mg per day.
Magnesium is used as a compound in many foods, especially in wholemeal products (for example wholegrain bread, wholemeal pasta, wholegrain rice, oatmeal, cornflakes), mineral water, especially medicinal water, tap water of sufficient water hardness, liver, poultry, fish, pumpkin seeds, sunflower seeds, chocolate , Cashew nuts, peanuts, potatoes, spinach, kohlrabi, berry fruits, oranges, bananas, sesame, sugar beet syrup, milk and dairy products.
Dangers and protective measures
The hazards of elemental magnesium depend heavily on temperature and particle size: compact magnesium is safe at temperatures below the melting point, while magnesium turnings and powders are highly flammable. Due to the large surface, the latter can easily react with the oxygen in the air. With very fine magnesium powder there is the danger of spontaneous combustion; Air-powder mixtures are even explosive. Phlegmatization is a hazard-reducing treatment in the processing of magnesium, such as metal powders in general. Molten magnesium also ignites spontaneously in the air. Even with many other substances, such as water and other oxygen-containing compounds, reacts fine-grained or heated magnesium. Magnesium melts therefore require permanent protection against the ingress of atmospheric oxygen. In practice, this is done by covering the melt by means of magnesium chloride-rich agents. Sulfur hexafluoride is also suitable as oxidation protection. The formerly usual covering with elemental sulfur is no longer practiced because of the strong annoyance caused by sulfur dioxide.
In magnesium fires, temperatures up to about 3000 ° C occur. Under no circumstances may common extinguishing agents such as water, carbon dioxide, foam or nitrogen be used as magnesium reacts violently with these. When water enters into a magnesium fire, there is an acute danger of an oxyhydrogen reaction.
For the fire (metal fires) of a melt, the extinguishing principle of suffocation applies, ie the rapid oxygen displacement. In the simplest case by covering with dry sand, otherwise by applying a covering salt for magnesium melts. Extinguishing powder of fire class D, magnesia powder (magnesia usta / burned magnesia), if necessary also dry stainless gray castor chips are also suitable.
When using magnesium, therefore, all given safety instructions must be followed exactly. Under no circumstances should an explosive atmosphere be created (magnesium dust, hydrogen, aerosols and vapors of combustible cooling lubricants). The normal occupational safety measures, such as the prevention of ignition sources, must also be observed.
proof
The best way to detect magnesium is with Magneson II, Titangelb or Chinalizarin.
For detection with Magneson II (4- (4-nitrophenylazo) -1-naphthol), the stock substance is dissolved in water and made alkaline. Thereafter, a few drops of a solution of the azo dye Magneson II are added. In the presence of magnesium ions, a dark blue colored lake is formed. Other alkaline earth metals should be previously removed by precipitation as carbonates.
For detection with Titangelb (Thiazolgelb G), the stock substance is dissolved in water and acidified. It is then mixed with a drop of Titangelb solution and made alkaline with dilute sodium hydroxide solution. The presence of magnesium produces a bright red precipitate. Nickel, zinc, manganese and cobalt ions interfere with this detection and should first be precipitated as sulfides.
For detection with quinalizarin, the acid sample solution is mixed with two drops of the dye solution. Then dilute sodium hydroxide solution is added until the basic reaction. A blue stain or precipitate indicates magnesium.
As a detection reaction for magnesium salts and the formation of precipitates can be used with phosphate salt solutions. The heavy metal-free, with ammonia and ammonium chloride to pH 8 to 9 buffered sample solution is added to it with disodium hydrogen phosphate solution. A white, acid-soluble turbidity due to magnesium ammonium phosphate MgNH4PO4 indicates magnesium ions:
From ammoniacal solution Mg2 + can also be detected with oxine as a sparingly soluble yellow-greenish compound. This proof is suitable for the cation separation process.
Connections
In compounds, magnesium occurs almost exclusively as a divalent cation with the oxidation state 2.
Oxides and hydroxides
Magnesium oxide (magnesia) forms colorless crystals in the sodium chloride structure. In nature, it occurs as a volcanic mineral periclas. They are white to gray, inclusions also dark green, glossy glassy regular crystals. It is added to foods as an acidity regulator or release agent and also has various engineering applications in laboratories and in industry. In medicine it is used for substitution therapy.
Magnesium hydroxide is a colorless, strongly basic salt that occurs naturally as a mineral brucite. It has a trigonal crystal structure in the space group P3m1 (room group no. 164) and is used as edible oil additive (for setting sulfur dioxide), as a flocculant for wastewater treatment, as a flame retardant in thermoplastics (polyolefins, polyvinyl chloride) and elastomers and as an additive in cleaning agents used. In medicine, it is used as an antacid for the neutralization of gastric acid and as a mild laxative used.
Magnesium peroxide is a finely powdered, colorless compound that has a pyrite crystal structure in the space group Pa3 (space group number 205). It resembles calcium peroxide and releases oxygen by controlled reaction with aqueous solutions. It has various applications in agriculture, pharmacy and cosmetics.
halides
Magnesium chloride is highly hygroscopic and occurs in nature in the mineral bishopite (MgCl2 · 6 H2O), as double salt carnallite (KMgCl3 · 6 H2O), in seawater and in salt lakes. It crystallizes in the trigonal crystal system in the space group R3m (space group number 166). In food technology, it is used as an acidity regulator, consolidator, flavor enhancer, carrier or release agent. Magnesium chloride hexahydrate, as a thermal battery, can store and release heat energy.
Magnesium fluoride forms colorless crystals that crystallize tetragonally in the rutile structure in the space group P42 / mnm (space group number 136) space group. Its optical properties and its chemical stability make it an important material for optical applications.
Magnesium bromide and magnesium iodide are also hygroscopic salts that have a trigonal crystal structure in the space group P3m1 (space group number 164).
Other inorganic compounds
Magnesium carbonate occurs in nature in large quantities as magnesite (bitterspar). It crystallizes trigonal in the space group R3c (space group number 167). In the food industry, it is added as an acidity regulator, carrier or release agent. It is used for climbing and gymnastics and is also known as Magnesia and Chalk. The athletes then dry themselves in the palms before starting to practice so that their skin does not stick too tightly when grasping the bars of bars or the iron bars of horizontal bar or barbell. It also has medical and industrial applications.
Magnesium nitrate is a colorless, hygroscopic salt that is readily soluble in water. The hexahydrate (Mg (NO3) 2.6 H2O) has a monoclinic crystal structure with the space group P21 / c (space group number 14). It is used as fertilizer, latent heat storage (as hexahydrate) or in the ceramics industry.
Magnesium sulfate heptahydrate (Mg (SO4) · 7 H2O) is known as Mineral Epsomit. It forms colorless crystals that form a rhombic pseudotetragonal crystal lattice. The crystals often bloom in fibrous aggregates and form stalactites. It is used for fertilizers, as a drying agent and for medical application.
Magnesium phosphates (magnesium dihydrogen phosphate (Mg (H2PO4) 2), magnesium hydrogen phosphate (MgHPO4) and magnesium phosphate (Mg3 (PO4) 2)) are used in the industry as a ceramic raw material and as a flame retardant. In the food industry, they are used as a feed additive, laxative and food additive. Foods are added as an acidity regulator or release agent.
Spinel is a frequently occurring mineral from the mineral class of oxides and hydroxides with the idealized chemical composition MgAl2O4 and is thus chemically a magnesium aluminate. It crystallizes isotypically with magnetite in the cubic crystal system in the space group Fd3m (space group number 227).
Dolomite is a very common mineral from the mineral class of carbonates and nitrates with the chemical composition CaMg [CO3] 2 and is thus chemically a calcium-magnesium carbonate. It crystallizes in the trigonal crystal system in the space group R3 (space group number 148).
Magnesium hydride can be used as hydrogen and energy storage. Hydrogen released from magnesium hydride can produce a metal foam with interesting properties that is lighter than water.
Other interesting crystalline magnesium compounds include, for example, magnesium diboride, magnesium carbide, magnesium nitride, magnesium sulfide, magnesium silicide, magnesium germanide, magnesium metasilicate, magnesium titanium oxide and magnesium polonide.
organomagnesium
Magnesium organyls are organometallic compounds in which a bond between magnesium and carbon exists. Among the organomagnesium Grignard compounds (R-Mg-X) are by far the most important. Binary magnesium organyls and alkenylmagnesium halides play a significantly subordinate role.
Organylmagnesiumhalogenide
Organylmagnesium halides (usually called Grignard compound) are obtained in a direct process by the reaction of organyl halides with magnesium turnings. Grignard compounds are in solution in the Schlenk equilibrium. They react under halogen-organyl substitution to elemental organyls:
General:
z. For example:
or by addition of organyls with multiple bond systems:
General:
z. B .:
Binary magnesium organyls (R2Mg, also called magnesium diorganyls) can be produced in various ways:
by transmetalation, for example of mercury diorganylene:
by dismutation in the shift of the Schlenk equilibrium with the aid of 1,4-dioxane:
Magnesacycles (cyclic alkanes with a magnesium in the ring) can also be prepared with 1,4 dioxane.
by metathesis of Grignard compounds with lithium organyls
by hydromagnesization (addition of MgH2 to 1-alkenes):
by the addition of elemental magnesium to C = C double bonds in some unsaturated hydrocarbons such as 1,3-butadiene or anthracene (metal addition). For example, the reaction of 1,3-butadiene in tetrahydrofuran at room temperature is possible:

The produced magnesium butadiene, also called (2-butene-1,4-diyl) magnesium, can serve as a source of butadiene anions in further syntheses. Similarly, the orange-yellow magnesium anthracene is shown. Magnesium anthracene can then be used as a catalyst for the hydrogenation of magnesium.
alkenylmagnesium
Alkynes react with alkenes to give alkenylmagnesium halides in the course of so-called carbomagnesation:

Other organic compounds
Magnesium hydrogen citrate and trimagnesium dicitrate are magnesium salts of citric acid. Magnesium citrate is used as a medicine.
Magnesium monoperoxyphthalate is a disinfectant for surface disinfection.
Magnesium stearate is the magnesium salt of stearic acid and belongs to the lime soaps. It consists of one magnesium ion and two long-chain stearate ions.
Magnesium price
Magnesium prices -> prices for strategic metals
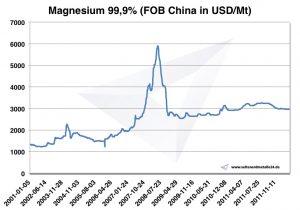
Chart Magnesium 2001-2011













