Tantalum, Ta, atomic number 73
Tantalum Price, Occurrence, Extraction and Use
Tantalum [ˈtantalum] is a chemical element with the symbol Ta and the atomic number 73; in the periodic table it is in the fifth subgroup or vanadium group. It is a rarely occurring, ductile, graphite-gray, shiny transition metal. Tantalum is mainly used for capacitors with high capacitance and at the same time small size. Since the metal is non-toxic and inert to body fluids, it is also used for implants, for example as a bone nail.
The element was discovered in 1802 by Anders Gustav Ekeberg in a Finnish columbite ore. He separated a very stable oxide (tantalum (V) oxide) that did not dissolve in any acid. It is named after Tantalus, a figure from Greek mythology. According to Ekeberg, the reason for this name is that the very permanent oxide "has to languish and cannot quench its thirst, like Tantalus in the underworld".
At almost the same time, Charles Hatchett found something very similar in a Colombian ore columbium. The two elements were believed to be identical until 1844, when Heinrich Rose recognized that two different elements were present in the columbite ores, namely tantalum and columbium. He called the latter niobium.
After the discovery of the new element, various chemists tried to represent tantalum in elemental form. The first to produce elemental tantalum by reducing tantalum fluoride with potassium was Jöns Jakob Berzelius in 1815. However, like the tantalum represented by Rose, its metal was only 50% tantalum. In 1902, Henri Moissan tried to produce tantalum in an electric furnace, but the carbon it contained made his product very hard and brittle.
Werner von Bolton was the first to produce pure, ductile tantalum in 1903. He achieved this by reducing the glowing oxides in a vacuum and by melting impure tantalum metal in a vacuum and using an electric flame arc.
The first application of the new element was as a filament in light bulbs. The reason for switching from the previously used osmium to tantalum was that it is easier to process and has a higher possible usable temperature of up to 2300 ° C. It was later replaced by tungsten, which has an even higher melting point and thus enables a light spectrum that is closer to that of sunlight and a higher light output.
In 1922 a new application was found for tantalum with its use in rectifiers and a year later in radio tubes.
occurrence
Tantalum is a rare element on earth with a content of 2 ppm in the continental crust and 8 ppm in the earth's shell. The frequency is comparable to that of arsenic and germanium. Within the group, the frequency decreases by a power of ten. Tantalum does not occur naturally, but only in the form of its compounds in various minerals. Due to the similarity of the two elements, tantalum ores always contain niobium and vice versa (socialization). The most important minerals are those of the columbite and tapiolite series, in which various minerals with the general formula (Mn, Fe2+) (Nb, Ta)2O6 be summarized. Tantalum columbites are also called tantalite designated. Examples of minerals containing tantalum in this series are ferrotapiolite (Fe2+, Mn2+) (Ta, Nb)2O6 and manganese tantalite MnTa2O6. These ores are often referred to as coltan. Less common minerals are microlite or thoreaulite.
The most important producing countries of tantalum ores in 2007 were Australia with 850 tons and Brazil with 250 tons. Coltan is also found in Canada and various African countries such as Ethiopia, Mozambique and Rwanda. The deposits in the east of the Democratic Republic of the Congo, which were fiercely contested in the Congo War 1996-2008, became known in the media.
Extraction and presentation
Since tantalum and niobium are always present together in the ores used for tantalum extraction, they must be separated for recovering the pure metals. This is complicated by the great similarity of the two elements.
The first method of separation was developed by Jean Charles Galissard de Marignac in 1866. He used the different solubility of the two elements in dilute hydrofluoric acid. Tantalum forms the slightly soluble K2TaF7, Niobium the well-soluble K3NbOF5 · 2 H2O.
The process used technically today is based on extraction and uses the different solubility of complex fluorine salts in water and certain organic solvents. The ore mixture is first dissolved in concentrated hydrofluoric acid or mixtures of hydrofluoric and sulfuric acid. The complex fluorides [NbOF5]2− and [TaF7]2−. After the insoluble constituents have been filtered off, separation can be carried out by liquid-liquid extraction with the aid of methyl isobutyl ketone. If methyl isobutyl ketone is added to the solution, the niobium and tantalum complexes pass into the organic phase, while other elements, such as iron or manganese, remain in the aqueous phase. When water is added to the separated organic phase, only the niobium complex dissolves in it, the tantalum remains in the methyl isobutyl ketone.
With the help of potassium fluoride, tantalum can be converted into a poorly soluble K2[TaF7] be felled. The reduction to elemental tantalum is usually done by sodium.
![\ mathrm {K_2 [TaF_7] + 5 \ Na \ longrightarrow Ta + 5 \ NaF + 2 \ KF}](https://upload.wikimedia.org/wikipedia/de/math/4/0/8/4082df7e6f3cde8acd65ddf4230284e5.png)
Reduction with sodium
A possible alternative to extraction is fractional distillation. The different boiling points of the two chlorides niobium pentachloride and tantalum pentachloride are used for this purpose. These can be extracted from the ores with chlorine and coke at high temperatures. After the separation, the tantalum chloride is also reduced to the metal with sodium.
In addition to the columbite-tantalite ores, slag from tin smelting is an important source for tantalum extraction (contains a few percent tantalum).
Physical Properties
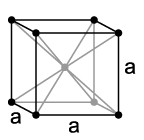
Crystal structure of tantalum,a = 330,3 pm
Tantalum is a distinctly purple-gray, steel-hard (Vickers hardness: 60–120 HV), high-melting heavy metal that is similar to niobium in most of its properties. It crystallizes in a body-centered cubic crystal structure. With a melting point of around 3000 ° C, tantalum has the highest melting point of all elements after tungsten, carbon and rhenium. If only a small amount of carbon or hydrogen is stored in the metal, the melting point increases significantly. With a melting point of 3880 ° C, tantalum carbide has one of the highest melting points of all substances.
Tantalum becomes a superconductor below a transition temperature of 4,3 Kelvin.
While pure tantalum is ductile and can be stretched considerably (tensile strength: 240 MPa), even small amounts of carbon or hydrogen added to it change the mechanical strength significantly. The material becomes brittle and difficult to process. This effect is used to produce tantalum powder. In technology it is loaded with hydrogen and thus embrittled, then comminuted accordingly and baked out or freed from hydrogen again at a higher temperature.
Chemical properties
Tantalum is a base metal and reacts at high temperatures with most non-metals, such as oxygen, the halogens or carbon. At room temperature, however, the metal is protected by a thin layer of tantalum (V) oxide and thus passivated. A reaction only takes place from a temperature of around 300 ° C.
In most acids tantalum is not soluble because of the passivation, even aqua regia can not dissolve the metal. Tantalum is attacked only by hydrofluoric acid, oleum (a mixture of sulfuric acid and sulfur trioxide) and molten salts.
isotope
There are a total of 30 isotopes and 26 core isomers of 155Ta up 185Ta known. Natural tantalum consists almost exclusively (99,988%) of the isotope 181Ta. 0,012% of the core isomer is also present 180mTa before. Although this can theoretically be radioactive, no decay has been observed so far. The half-life must therefore exceed 1 · 1015 Years.
Usage
Most of the tantalum (worldwide annual production volume 1.400 t) is used for very small capacitors with high capacitance. In 2007, 60% of the tantalum was used in the manufacture of capacitors. These tantalum electrolytic capacitors are used everywhere in modern microelectronics, for example for cell phones and in automobile construction. The effect is based on the tantalum oxide layer on the surface of the wound tantalum foil, which is still stable and reliably insulating even in a very thin version. The thinner the layer between the electrodes, the higher the capacity with the same foil surface; tantalum oxide also has an extremely high permittivity, which also increases the capacity.
Since tantalum is non-toxic and does not react with body tissue or fluids, elemental tantalum is used for medical implants and instruments. For example, bone nails, prostheses, brackets and jaw screws are made from tantalum. In addition, it is an X-ray contrast medium that is little used due to its high costs.
In the chemical industry, tantalum is used because of its durability. It serves as a lining material for reaction vessels and is used for heat exchangers and pumps. For these purposes, pure tantalum is usually not used, but alloys that contain 2,5–10% tungsten. These are more stable and resistant than pure tantalum. At the same time, the desired ductility is retained. Other uses are laboratory equipment, spinnerets and the cathodes of electron tubes. Here, tantalum benefits from the fact that it is able to absorb up to 800 parts by volume of gases at 740 ° C (getter effect), which ensures a high vacuum in the tubes.
Superalloys, which are used in the construction of turbines and aircraft engines, contain up to 9% tantalum. Adding 3–4% tantalum to a nickel superalloy increases the strength of the material at high temperatures.
safety instructions
Handling tantalum and its compounds normally does not cause problems under laboratory conditions. Elementary tantalum and tantalum compounds are not toxic. However, there are vague indications that some tantalum compounds are carcinogenic. Tantalum powder and dust - like other finely divided metals - pose a high risk of fire and explosion.
Connections
Tantalum (V) oxide Ta2O5 is a white powder used to make high-refractive glasses and special crystal materials.
Tantalum carbide TaC, with its melting temperature of 3880 ° C and a hardness almost that of a diamond, serves as a protective layer on high-temperature alloys in engines and cutting tools.
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, atomic number | Tantalum, Ta, 73 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series | Transition metals | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 5, 6, d | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | wheat | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-25-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth shell | 8 ppm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| atomic mass | 180,9479 u | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 145 (200) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 138 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electron configuration | [Xe] 4f14 5d3 6s2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. ionization | 761 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. ionization | 1500 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | fixed | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| crystal structure | cubic body-centered | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 16,65 g / cm3 (20 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 6,5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( = 1,8 10−4) = 1,8 10−4) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| melting point | 3290 K (3017 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 5731 K (5458 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 10,85 · 10−6 m3/ mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 735 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| heat of fusion | 36 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| speed of sound | 3400 m / s at 293,15 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 140 J / (kg · K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 7,61 · 106 A / (V · m) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thermal conductivity | 57 W / (m K) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| oxidation states | 5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| normal potential | −0,81 V (½ Ta2O5 + 5 H.+ + 5 e- → Ta + 2½ H2O) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electronegativity | 1,5 (Pauling scale) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| isotope | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Tantalum prices
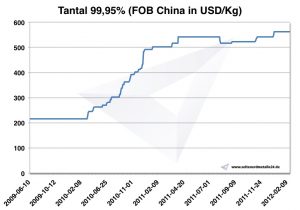
Chart Tantalum 2009-2012
Current tantalum prices


