Germanium, Ge, atomic number 32
Germanium price, occurrence, extraction, use
Germanium (from Latin Germania 'Germany', after the country in which it was first found) is a chemical element with the element symbol Ge and atomic number 32. In the periodic table it is in the 4. Period and in the 4. Main group (14, IUPAC group, p-block and carbon group). It was on 6. February 1886 first detected in mineral argyrodite.
History
When Xnumx Dmitri Mendeleev designed the periodic table, he discovered a gap below the silicon and postulated a hitherto unknown element, which he called Eka silicon. Mendeleev made predictions about the properties of Eka-silicon and its compounds, which were rejected by science, however.
1885 found Clemens Winkler (1838-1904), a chemist at the Bergakademie Freiberg, when working with the newly discovered mineral argyrodite, that his quantitative analysis always delivered a deficit of around seven percent. As often as the analysis was repeated, the shortfall remained approximately constant and Winkler finally guessed that the mineral contained a hitherto unknown element. After four months of work, he finally succeeded in 6. February 1886 the isolation of a white sulfide precipitate, which could be reduced in the hydrogen stream to a metallic powder. Following the previously discovered planet Neptune, Winkler initially wanted to call the new element Neptunium. Since this name had already been used for another suspected element, he named it after the discovery Germanium. Winkler first suggested that the Germanium was the Eka stibium postulated by Mendeleev, while Mendeleev initially wanted to classify it as Eka cadmium rather than Eka silicon. After further properties were determined, it was confirmed that it was probably the predicted element Eka-silicon. Mendeleev had derived its properties from his periodic table, so that this find contributed to the recognition of the periodic table:
The origin and etymology of the name germanium could also stem from a semantic misunderstanding in connection with its predecessor element gallium, because there are two theories for the naming of gallium. After the first, the French chemist Paul Émile Lecoq de Boisbaudran named the element after Gaul, the Latin name of his native France. The second is also the Latin word gallus (cock) as the source of the name, which means le coq ("the cock") in French. Paul Émile Lecoq de Boisbaudran would have named the new element after his own name. Winkler assumed that the previous element Gallium was named after the French explorer's nationality. So he called the new chemical element "Germanium" in honor of his country (Latin Germania for Germany).
occurrence
Germanium is widespread but occurs only in very low concentrations; Clarke value (= average content in the earth's crust): 1,5 g / t. In nature, it usually occurs as a sulfide (thiogermanate) and is often found as a companion in copper and zinc ores (Mansfeld copper shale). The most important minerals are argyrodite 

Extraction and production
According to USGS, 2014's annual production was estimated at 165 t, of which 120 t was in China. The price for 1 kg Germanium was 2014 about 1.900 USD. According to the EU, the price of 2003 was 300 USD / kg, rising to 2009 USD by 1.000.
For the representation of germanium, in particular the flue gases (contain gemanium oxide ( 

Features
elemental germanium
Germanium is in the periodic table in the series of semi-metals, but is classified by recent definition as a semiconductor. Elemental germanium is very brittle and very stable in air at room temperature. It is oxidized to germanium (IV) oxide (GeO2) only after strong annealing in an oxygen atmosphere. GeO2 is dimorphic and is converted at 1033 ° C by the rutile modification (CN = 6) into the ß-quartz structure (CN = 4). In powder form, it is a flammable solid and can easily be ignited by brief exposure to an ignition source and continues to burn after removal. The risk of ignition is greater, the finer the substance is distributed. In a compact form, it is not flammable. Germanium is bivalent and tetravalent. Germanium (IV) compounds are the most stable. Of hydrochloric acid, potassium hydroxide solution and dilute sulfuric acid germanium is not attacked. In alkaline hydrogen peroxide solutions, concentrated hot sulfuric acid and concentrated nitric acid, however, it is dissolved to form germanium dioxide hydrate. According to its position in the periodic table, its chemical properties are between silicon and tin.
Germanium is one of the few substances that has the property of density anomaly. Its density is lower in solid state than in liquid state. Its band gap is about 0,67 eV at room temperature.
Germanium wafers are considerably more fragile than silicon wafers.
Usage
Electronics
As a semiconductor, germanium in single crystal form was the leading material in the electronics center of the 20. Especially for the production of the first germanium diodes and bipolar transistors available on the market until it was displaced by silicon in these areas. Today, applications are found in high-frequency technology (for example as silicon germanium compound semiconductor) and detector technology (for example as an X-ray detector). Gallium arsenide (GaAs) solar cells are sometimes made of germanium wafers. The lattice constant of germanium is very similar to that of gallium arsenide, so that GaAs epitaxially grows on germanium single crystals.
Glasses and fibers
Its second main application is in the field of infrared optics in the form of windows and lens systems made of polycrystalline or monocrystalline germanium and optical glasses with infrared transmittance, so-called chalcogenide glasses. Applications include military and civilian night vision devices as well as thermal imaging cameras.
Other major uses are in the production of optical fibers and polyester fibers: In modern glass fibers for telecommunications, germanium tetrachloride is used in the chemical vapor deposition to generate an accumulation of germanium dioxide in the inner fiber core. This results in a higher refractive index in the core compared to the fiber cladding, whereby the guidance of the light waves is ensured. In polyester chemistry, germanium dioxide is used as a catalyst in the production of certain polyester fibers and granulates, especially for recyclable PET bottles (PET = polyethylene terephthalate).
Nuclear medicine and nuclear technology
68Ge is used in the Gallium 68 generator as a parent nuclide for the production of Gallium-68. Similarly, 68Ge is used as a source for detector calibration in positron emission tomography.
As a high-purity single crystal germanium is used as a radiation detector.
Germanium in nutritional supplements
The substance Bi (carboxyethyl) germanium sesquioxide (Ge-132) has been touted as a dietary supplement for use in a number of diseases including cancer, chronic fatigue syndrome, immunodeficiency, AIDS, hypertension, arthritis and food allergies. Positive effects on the course of the disease have not yet been scientifically proven.
According to European Directive 2002 / 46 / EC on the approximation of the laws of the Member States relating to food supplements, germanium should not be used in food supplements. In many EU countries that have already harmonized their national legislation, including Germany and Austria, the addition of germanium as a mineral source in dietary supplements is therefore not permitted.
The competent authorities expressly warn against the consumption of Ge-132, as serious health effects and deaths can not be ruled out.
Medicinal use of germanium
Therapeutic efficacy of the antineoplastic substance spirogermanium in cancer has not been demonstrated. Approved finished medicinal products with the active ingredient spirogermanium do not exist. In Germany, Germanium-containing pharmaceutical preparations (formulations), apart from homeopathic dilutions from D4, are considered questionable. Their production and delivery are therefore prohibited. Germanium metallicum is available in the form of homeopathic medicines. As part of homeopathic preparations di-potassium germanium citrate lactate is described.
physiology
Germanium and its compounds have a relatively low toxicity. Traces of germanium are included in the following foods: beans, tomato juice, oysters, tuna and garlic. It is not an essential trace element according to the state of the art. There is no known biological function for germanium. A possible influence on carbohydrate metabolism has been discussed. There are no germanium deficiency diseases known.
toxicity
In the past, poisoning with germanium in humans occurred only after the intake of inorganic germanium compounds as a dietary supplement. The first symptoms are loss of appetite, weight loss, fatigue and muscle weakness. This is followed by malfunction of the kidney, up to kidney failure, which can be lethal to the patient. Peripheral neuropathy as a consequence of disease is also reported.
Transient neurotoxic side effects when taking spirogermanium in clinical trials are reported. Spiro-germanium was tested as a cytostatic in the 1980 years. Data from studies on healthy volunteers are not available.
From animal experiments it is known that germanium has a low acute oral toxicity. The symptoms of acute poisoning with large doses of germanium compounds include:
- Dilation of the blood vessels (artectasia)
- Ptosis
- cyanosis
- Tremor
Finally, respiratory paralysis leads to the death of the experimental animals. Symptoms of chronic or subchronic poisoning with inorganic germanium compounds are:
- weight loss
- Organ changes (mass of organs)
- Progressive neuropathy
- kidney damage
Organic germanium compounds showed lower toxicity, but resulted in the experimental animals to weight loss and a decrease in the number of red blood cells. There are only limited data on the teratogenic effects of germanium. Sodium germanate was tested in rats as non-carcinogenic.
The mechanism of toxicity of germanium is not yet fully understood. However, specific pathological effects on the mitochondria of kidney and nerve cells have been observed.
interactions
It is also discussed whether germanium may show interactions with silicon in bone metabolism. It can block the action of diuretics and decrease the activity of a number of enzymes, such as dehydrogenases. In animal experiments, mice show increased hexabarbital-induced sleep duration when additionally treated with germanium compounds. This suggests that cytochrome P450 activity is also restricted. There are reports of organic germanium compounds blocking the detoxification enzyme glutathione-S-transferase.
Bioavailability and metabolism
Germanium is easily absorbed by the body when ingested. It is distributed over the entire body tissue, especially in the kidneys and the thyroid gland. In contrast to inorganic germanium compounds, organogermans do not accumulate in the human body. However, there are only a few studies on germanium metabolism.
It is essentially excreted in the urine. Excretion via bile and feces also takes place.
Connections
Germanium forms Ge (II) - u. more stable Ge (IV) compounds, only a few have technical significance.
Of the germanium halides are also Ge (II) - u. Ge (IV) representative known. Germanium tetrachloride, (GeCl4), a liquid with a boiling point of 83 ° C, forms on exposure to hydrogen chloride to germanium oxides and is an important intermediate in germanium recovery. High-purity GeCl4 is used in the production of quartz glass optical fibers to produce a high-purity germanium (IV) oxide layer on the inside of the quartz fibers. To produce high-purity germanium layers, it is also possible to use the disproportionation of germanium (II) iodide to form germanium and germanium (IV) iodide:
Germanates are compounds of germanium derived from its oxide. In almost all germanium-containing minerals germanium is present as germanate.
Germans are called the hydrogen compounds of germanium, which form a homologous series of different long chain molecules. Monogerman or germanium hydride (GeH4) is a gas used in the semiconductor industry for epitaxy and doping.
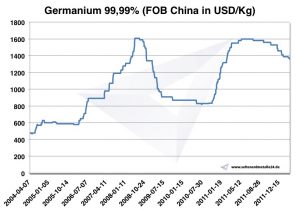
Chart Germanium 2004-2011

