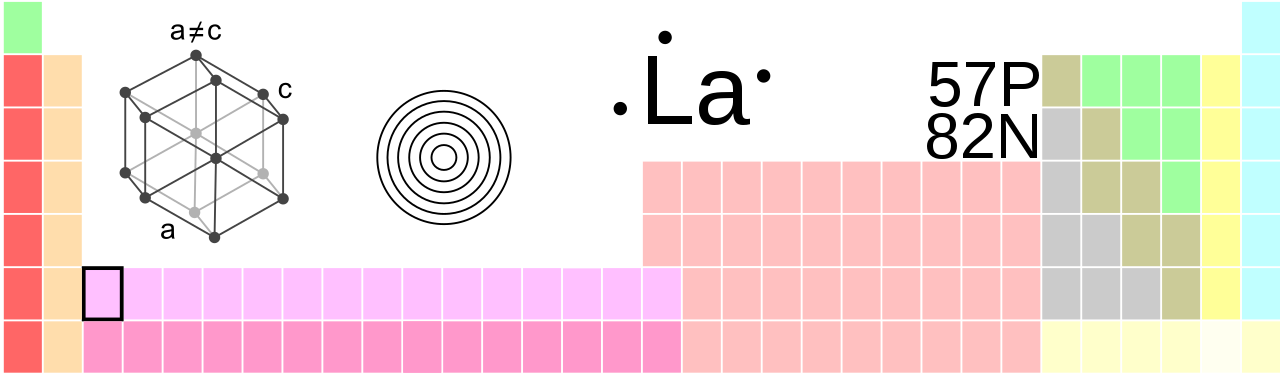
Lanthanum, La, atomic number 57
General
Lanthanum [lantaːn] is a chemical element with the element symbol La and the atomic number 57. It belongs to the transition metals as well as the metals of the rare earths, in the periodic table stands it in the 6. Period and the 3. Subgroup (group 3) or scandium group. Mostly it is also counted to the Lanthanoiden, even if the f-shell of the element is unoccupied.
Lanthanum (Greek: λανθάνειν, lanthanein, "to be hidden") 1839 was discovered by the Swedish chemist and surgeon Carl Gustav Mosander. From a supposedly pure cerium nitrate he won by fractional crystallization Lanthanum sulfate.
Lanthanum occurs naturally only in chemical compounds associated with other lanthanides in various minerals. Mainly these are:
Monazite ((Ce, La, Th, Nd, Y) PO4)
Bastnäsit ((Ce, La, Y) CO3F)
Recovery
After a complex separation of the other lanthanide companion, the oxide is reacted with hydrogen fluoride to lanthanum fluoride. Subsequently, this is reduced to lanthanum with calcium to form calcium fluoride. The separation of remaining calcium residues and impurities takes place in an additional remelting in vacuo.
Features
The silvery white metal is malleable and ductile. There are three metallic modifications.
Lanthanum is undignified. It quickly becomes coated with a white oxide layer in the air, which reacts in the moist air to the hydroxide.
At temperatures above 440 ° C lanthanum burns to lanthanum oxide (La2O3). The formation of hydrogen in cold water is slow, in warm water a rapid reaction to the hydroxide. In dilute acids, lanthanum dissolves under evolution of hydrogen. With many elements it reacts directly in the heat, with halogens even at room temperature. Lanthanum and hydrogen form a black, water-sensitive unstoichiometric hydride.
Usage
Lanthanum is a component in misch metal. Pyrophoric flint materials contain 25 to 45 weight percent lanthanum. In addition, it finds use as a reducing agent in metallurgy. As a cast iron addition, it supports the formation of spheroidal graphite, as an alloying additive, it improves the oxidation resistance. Lanthanum admixtures reduce the hardness and temperature sensitivity of molybdenum.
High-quality cathodes for generating free electrons consist of lanthanum hexaboride as a substitute for tungsten wire. High-purity lanthanum oxide is used in the glass industry for the production of high-quality glasses with a high refractive index for the optics z. B. used for camera lenses.
With Cobalt:
The cobalt-lanthanum alloy LaCo5 is used as a magnetic material, lanthanum-doped barium titanate for the production of PTC thermistors (temperature-dependent resistors). In conjunction with cobalt, iron, manganese, strontium and others, it serves as a cathode for high-temperature fuel cells (SOFC). "Contaminated" lanthanum nickel (LaNi5) is used as hydrogen storage in nickel metal hydride storage batteries. In addition, it comes in carbon arc lamps for studio lighting and in film projection systems (historical application?) Before.
With titanium:
An alloying metal with material compositions of lanthanum and titanium, the effect is attributed to the fact that with chip-forming processing, the chip length is reduced. This should facilitate the processing of the metal.
In the field of medicine, corrosion-resistant and easily sterilizable instruments are produced from the alloying metal. This titanium-based metal alloy is said to be particularly well-suited for surgical tools and apparatus because the allergy propensity of using such metal alloy with titanium relative to other alloys should be low.
As lanthanum oxide
Production of glasses (Lanthanglas) with comparatively high refractive index, which in turn changes only slightly with the wavelength (low dispersion), for cameras, telescope lenses and for spectacle lenses
Production of crystal glass and porcelain stains. It replaces more toxic lead compounds with simultaneous improvement of chemical resistance (improvement of alkali resistance, "dishwasher safe")
Catalyst addition to zeolites during fluid catalytic cracking in the oil refinery
Production of ceramic condenser masses and silicate-free glasses
Component of glass polishers
Production of hot cathodes for electron tubes (also lanthanum borides)
As lanthanum carbonate
Drug for lowering the phosphate level in dialysis patients (so-called phosphate binder)
Lanthanum is classified as low in toxicity. A toxic dose is unknown. However, lanthanum powder is considered to be highly corrosive because it reacts very easily, for example by skin moisture, to basic lanthanum hydroxide (similar to the elements calcium and strontium). The lethal dose in rats is 720 mg.
| General | |
| Name, symbolOrder number | Lanthanum, La, 57 |
| Series | Transition metals |
| Group, period, block | 3, 6, d |
| Appearance | silvery white |
| CAS number | 7439-91-0 |
| Mass fraction of the earth's envelope | 17 ppm |
| Atomic | |
| atomic mass | 138,9055 u |
| atomic radius | 195 pm |
| Covalent radius | 207 pm |
| Elektronenkonf. | [Xe] 5d (1) 6s2 |
| 1. ionization | 538,1 KJ / mol |
| 2. ionization | 1067 KJ / mol |
| 3. ionization | 1850 KJ / mol |
| Physically | |
| Physical state | fixed |
| crystal structure | hexagonal |
| density | 6,17 g / cm3 (20 ° C) |
| magnetism | paramagnetic (χm = 5,4 * 10 (-5)) |
| melting point | 1193 K (920 C) |
| boiling point | 3743 K (3470 C) |
| Molar volume | 22,39 * 10 (-6) m (3) / mol |
| Heat of vaporization | 400 KJ / mol |
| heat of fusion | 6,2 KJ / mol |
| Electric conductivity | 1,626 * 10 (6) A / (V * m) |
| thermal conductivity | 13 W / (m * K) |
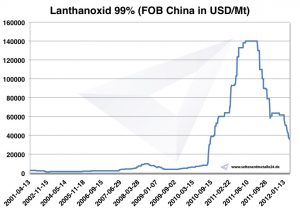
Lanthanum price