Sodium, Na, atomic number 11
Sodium Price, Occurrence, Extraction and Use
Sodium is a frequently occurring chemical element with the symbol Na and the atomic number 11. In the periodic table of the elements it is in the 3rd period and as an alkali metal in the 1st IUPAC group or 1st main group. Sodium is a pure element, the only stable isotope of which is 23Na.
 Elemental sodium was first obtained from sodium hydroxide by Humphry Davy in 1807 by fused-salt electrolysis and called sodium. This designation is used in the English and French language areas, derivatives of it in the Romance and partly also in Slavic languages. The German name sodium is derived from the Arabic نطرون, DMG naṭrūn, Natron, from the Egyptian netjerj. Sodium and its derivatives are used in Scandinavia, Dutch and some Slavic languages, except in the German language. In Japanese, sodium has the German-sounding name Japanese ナ ト リ ウ ム Natoriumu.
Elemental sodium was first obtained from sodium hydroxide by Humphry Davy in 1807 by fused-salt electrolysis and called sodium. This designation is used in the English and French language areas, derivatives of it in the Romance and partly also in Slavic languages. The German name sodium is derived from the Arabic نطرون, DMG naṭrūn, Natron, from the Egyptian netjerj. Sodium and its derivatives are used in Scandinavia, Dutch and some Slavic languages, except in the German language. In Japanese, sodium has the German-sounding name Japanese ナ ト リ ウ ム Natoriumu.
Under normal conditions, sodium is a waxy, silvery, highly reactive metal. Because of its strong reactivity, metallic (elemental) sodium is stored under inert conditions, mostly in paraffin oil or petroleum, for larger quantities in airtight steel drums.
Sodium is one of the ten most common elements in the earth's shell and occurs in numerous minerals in the earth's crust. Seawater contains a significant amount of sodium in the form of sodium ions.
History
In ancient times, the Egyptians coined the term netjerj (neter) for soda obtained from soda lakes. The Greeks adopted this word as Greek νίτρον nitron, the Romans as nitrium, the Arabs as natrun. In contrast to the elementary metal, sodium compounds have been known for a long time and have since been extracted from seawater or lakes, mined from underground deposits and traded. The most important sodium compound, table salt (sodium chloride), was obtained in mines or by drying seawater or salty spring water in salt pans. The salt trade was the basis of their wealth for many cities, and in some cases even shaped their names (Salzgitter, Salzburg). Place names such as Hallstatt, Hallein, Halle (Saale), Bad Hall, Bad Reichenhall, Schwäbisch Hall, Schweizerhalle or Hall in Tirol allude to the Germanic name for Saline (Hall). Other naturally occurring sodium compounds such as sodium carbonate (soda or baking soda) and sodium nitrate have also been extracted and traded since ancient times.
It was not until 1807 that Humphry Davy succeeded in producing elemental sodium through the electrolysis of molten sodium hydroxide (caustic soda) using voltaic columns as a power source. As he reported to the Royal Society in London on November 19, 1807, he won two different metals: he called the sodium contained in soda sodium, which is the name still used in French and English-speaking countries; the other metal he called potassium. In 1811 Berzelius suggested the current name sodium.
occurrence
 In the universe, sodium is 14th in frequency, comparable to calcium and nickel. The yellow sodium D line can be easily detected in the light emitted by many celestial bodies, including that of the sun.
In the universe, sodium is 14th in frequency, comparable to calcium and nickel. The yellow sodium D line can be easily detected in the light emitted by many celestial bodies, including that of the sun.
Sodium is the sixth most abundant element on earth, making up 2,36% of the earth's crust. Due to its reactivity, it does not occur in elemental form, but always in compounds, the sodium salts. Sea water is a great store of sodium. One liter of seawater contains an average of 11 grams of sodium ions.
Common sodium minerals are albite (called soda feldspar), NaAlSi3O8 and oligoclase (Na, Ca) Al (Si, Al) 3O8. In addition to these rock-forming minerals, which are part of the feldspars, sodium occurs in large salt deposits. Above all, there are large deposits of halite (sodium chloride, colloquially often called rock salt), which have arisen from the drying up of parts of the sea. These represent the most important source for the extraction of sodium and its compounds. Well-known German salt production sites include Salzgitter, Bad Reichenhall, Stade and Bad Friedrichshall.
In addition to the common sodium chloride, other compounds occur in nature. Sodium nitrate or sodium nitrate (also called Chile nitrate) NaNO3 is one of the few natural nitrate minerals. Because of its good solubility in water, it occurs only in particularly dry areas, such as the Atacama Desert in Chile. Before the Haber-Bosch process was invented, this was the most important raw material for many fertilizers and explosives.
Sodium carbonate Na2CO3 is also found naturally in several minerals. The best-known mineral is soda Na2CO3 · 10 H2O. It is mined in large quantities and used primarily in glass production.
There are also a large number of other sodium minerals (see also: Category: Sodium Minerals). A well-known one is cryolite (ice stone, Na3 [AlF6]), which in its molten state serves as a solvent for aluminum oxide in aluminum production. Since the only known cryolite deposit is mined in Greenland, cryolite is artificially produced.
Extraction and presentation

The large-scale production of sodium is carried out by fused-salt electrolysis of dry sodium chloride in a so-called Downs cell (patented in 1924 by James C. Downs). A eutectic salt mixture of 60% calcium chloride and 40% sodium chloride, which melts at 580 ° C., is used to lower the melting point. Barium chloride is also possible as an additive. A voltage of about seven volts is applied. To produce one kilogram of sodium, around 10 kWh of electricity are used during electrolysis, and around 12 kWh in the entire production process.
Formation of sodium on the cathode
Formation of chlorine on the anode
Overall response
The cylindrical electrolysis cell consists of a central graphite anode and a side cathode ring made of iron. Above the cell is a bell that collects and discharges the chlorine that has formed. The sodium collects above the cathodes and is removed from the cell through a cooled riser pipe. Calcium that has also formed crystallizes there and falls back into the melt.
The electrolysis of sodium chloride replaced the Castner process. The sodium was obtained by fused-salt electrolysis of sodium hydroxide. This had the advantage of the lower melting point of sodium hydroxide (318 ° C), but more electrical energy is required. Since the introduction of chlor-alkali fused-salt electrolysis, the price of sodium has fallen dramatically. In terms of volume, sodium is thus the cheapest light metal of all. The price, however, depends heavily on the electricity costs and the price of the chlorine that is also produced.
Physical Properties
 Sodium is a silvery white, soft light metal. In many properties it stands between lithium and potassium. The melting point of 97,82 ° C is between that of lithium (180,54 ° C) and that of potassium (63,6 ° C). This is similar with the boiling point and the specific heat capacity. With a density of 0,968 g · cm − 3, sodium is one of the specifically lightest elements. Of the elements that are solid at room temperature, only lithium and potassium have a lower density. With a Mohs hardness of 0,5, sodium is so soft that it can be cut with a knife.
Sodium is a silvery white, soft light metal. In many properties it stands between lithium and potassium. The melting point of 97,82 ° C is between that of lithium (180,54 ° C) and that of potassium (63,6 ° C). This is similar with the boiling point and the specific heat capacity. With a density of 0,968 g · cm − 3, sodium is one of the specifically lightest elements. Of the elements that are solid at room temperature, only lithium and potassium have a lower density. With a Mohs hardness of 0,5, sodium is so soft that it can be cut with a knife.
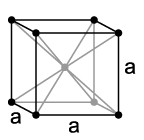
Like the other alkali metals, sodium crystallizes in the cubic crystal system in a body-centered lattice with the space group Im3m (space group no. 229) and two formula units per unit cell. Below 51 K it changes into a hexagonal closest packing of spheres with the lattice parameters a = 376 pm and c = 615 pm.
Sodium vapor consists of both individual metal atoms and dimers of the form Na2. At the boiling point, 16% of the atoms are in the form of dimers. The vapor is yellow and appears purple when viewed through.
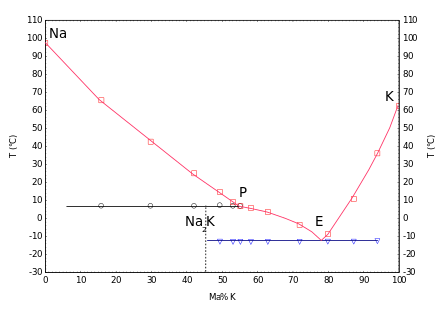
With potassium, liquid mixtures are formed in a wide range of concentrations at room temperature. The phase diagram shows an incongruent melting compound Na7K at 2 ° C and a eutectic at −12,6 ° C with a potassium content of 77% (mass fraction).
Chemical properties
 Like the other alkali metals, sodium is a very base element (normal potential: −2,71 V) and reacts easily with many other elements and sometimes with compounds. The reactions are particularly violent with non-metals, such as chlorine or sulfur, and occur with a bright yellow flame.
Like the other alkali metals, sodium is a very base element (normal potential: −2,71 V) and reacts easily with many other elements and sometimes with compounds. The reactions are particularly violent with non-metals, such as chlorine or sulfur, and occur with a bright yellow flame.
The otherwise reactive oxygen is a specialty. Sodium and oxygen do not react directly with one another without the presence of water at room temperature or when heated. In a completely anhydrous oxygen atmosphere, sodium can even be melted without reacting. If, on the other hand, there are traces of moisture, it burns easily to form sodium peroxide.
Reaction of sodium with oxygen
The strongly exothermic reaction of sodium with water
 Sodium reacts with water to form sodium hydroxide, forming hydrogen. High-speed recordings of the reaction of alkali metals with water suggest a Coulomb explosion.
Sodium reacts with water to form sodium hydroxide, forming hydrogen. High-speed recordings of the reaction of alkali metals with water suggest a Coulomb explosion.
Reaction of sodium with water
In alcohols, sodium is converted into sodium alcoholates with the formation of hydrogen. It often melts due to the high heat of reaction. If the sodium is finely distributed and the resulting large reaction surface area, the reaction can be explosive and ignite the hydrogen.
Reaction of sodium with ethanol
If sodium comes into contact with chlorinated compounds such as dichloromethane, chloroform, carbon tetrachloride, a rapid and exothermic reaction occurs with the formation of sodium chloride.
Sodium dissolved in liquid ammonia
Sodium dissolves in liquid ammonia with a blue color. The color is based on free electrons that are released into the solution by the sodium. The solution also conducts electrical current and is diluted paramagnetic. The anion of sodium, the sodium ion, for example in the form of potassium (2.2.2-cryptand) natride (K + (C222) Na−) can be represented in a similar way. It is a very powerful reducing agent.
isotope
A total of 19 isotopes and 3 further core isomers from 18Na to 37Na are known of sodium. Of these, only one occurs naturally, the isotope 23Na. This makes sodium one of 22 pure elements. The longest-lived artificial isotopes are 22Na, which converts to 2,602Ne with a half-life of 22 years under beta-plus decay (β +) and 24Na, which also decays to 14,957Mg with a half-life of 24 hours under beta decay. These are used as tracers in nuclear medicine. 22Na can be produced by irradiating magnesium or aluminum targets with protons from a cyclotron for several weeks.
All other isotopes and isomers only have short half-lives of seconds or milliseconds.
Usage

Sodium is the most widely used alkali metal. It is used for various purposes both technically and in the laboratory. In school lessons and during experimental lectures, sodium can be used to produce hydrogen with the aid of a sodium spoon and water. A number of sodium compounds are made from some of the sodium. These are, for example, sodium peroxide used as a bleaching agent and the strong base sodium amide. These do not occur naturally and cannot be obtained directly from sodium chloride. Sodium cyanide and sodium hydride are made from sodium. Since sodium influences the solidification structure, it can be used as an addition to aluminum-silicon alloys (refining process according to Aladár Pácz).
Catalyst
Sodium catalyzes the polymerization of 1,3-butadiene and isoprene. Hence it was used for the production of artificial rubber. Plastic made using sodium as a catalyst, known as buna, was the world's first man-made rubber. From 1937 it was produced in the Buna works (named after butadiene and sodium) in Schkopau.
Coolant
Since sodium with a thermal conductivity of 140 W / (m · K), which is well above that of steel (15 to 58 W / (m · K)), has good heat transfer properties and also has a low melting point with a large liquid range at the same time it is used as a coolant to cool the exhaust valves in internal combustion engines, which are subject to high thermal loads. For this purpose, the valve stems are made hollow and partly filled with sodium. During operation, the sodium melts and sloshes back and forth between the hot and cold sides. The heat is transported away from the red-hot valve disc.
Fast breeders are cooled with molten sodium. In such breeder reactors, the fast neutrons produced during nuclear fission must not be slowed down between the fuel rods, as in other types of reactor. Therefore, water, which acts as a braking agent (moderator), must not be used for cooling. The heat is then passed on to the steam generator for turbine operation via a secondary sodium circuit.
Light generation
Sodium vapor lamps use the characteristic yellow light that sodium vapor emits during an electrical discharge. Due to their high luminous efficacy, they are often used for street lighting.
Reducing agent
Some metals, such as titanium, zirconium, tantalum or uranium, cannot be obtained by reduction with carbon because stable and non-separable carbides are formed. In addition to some other elements, in particular aluminum and magnesium, sodium is therefore used as a reducing agent. Another element that sodium is used to make is potassium. Since potassium is a very base element, it cannot be obtained by reduction with carbon. A theoretically possible production by electrolysis is technically not possible due to the good solubility of potassium in a potassium chloride melt.
Sodium plays an important role as a reducing agent in organic synthesis. For a long time, the most important technical sodium application was the production of tetraethyl lead from chloroethane. This was an important anti-knock agent that was added to gasoline. For environmental reasons, the use of tetraethyl lead has been severely restricted or banned entirely. Therefore, the consumption of sodium decreased. Otherwise, sodium is used in other reactions such as the Birch reduction and pinacol coupling. However, these are more of interest on a laboratory scale.
desiccant
Since sodium also reacts with traces of water, freshly pressed sodium wire can be used to dry organic solvents such as diethyl ether or toluene. This method is not suitable for halogen-containing solvents (examples: methylene chloride, chloroform) because of the violent reaction with the chlorine atom.
Sodium-potassium alloys are liquid at room temperature. These are used for heat transfer and dehalogenation in organic synthesis. Na-K is well suited for drying some well-predried solvents in order to achieve particularly low residual water contents.
Electrical conductor
During the 1960s, polyethylene jacketed sodium cables were experimented with. Because of the lower conductivity, a hypothetical sodium cable would have a 75% larger diameter.
proof
 The qualitative detection and the quantitative determination are carried out by atomic spectroscopy through the intense yellow color of the flame or, more precisely, through the double Na line at 588,99 nm and 589,59 nm.
The qualitative detection and the quantitative determination are carried out by atomic spectroscopy through the intense yellow color of the flame or, more precisely, through the double Na line at 588,99 nm and 589,59 nm.
The detection of sodium in a purely chemical way is very difficult. Since almost all sodium compounds are readily soluble in water, classic precipitation reactions and gravimetric determinations are hardly possible. Exceptions are the yellow sodium magnesium uranyl acetate NaMg (UO2) 3 (CH3COO) 9 · 9 H2O and the colorless sodium hexahydroxoantimonate Na [Sb (OH) 6], both of which are sparingly soluble. A precipitation reaction with the sulfate-bismuth double salt 3Na2SO4 · 2Bi2 (SO4) 3 · 2H2O is possible. Since sodium ions are colorless in aqueous solution, color reactions are rarely carried out. Therefore, apart from ion chromatography, only spectroscopic methods are of practical importance.
physiology
Sodium is one of the elements that are essential for all animal organisms. In the animal organism, sodium - together with chlorine - is the ninth most common element and is the third most common inorganic ion after calcium and potassium. Physiologically it is therefore one of the mass elements. Sodium occurs in living things in the form of Na + ions.
With an average body weight of 70 kg, the human body contains around 100 g of sodium as Na + ions. Two thirds of this is available as NaCl and one third as NaHCO3. Since it makes up 90% of the extracellular electrolytes in the human body, the sodium concentration determines the volume of the interstitial fluid via the vessel volume.
Recommended and Actual Sodium Intakes
According to the DA-CH reference values, the estimated value for the minimum intake of sodium is 550 mg / day for adults. However, various organizations have made recommendations in particular for a maximum intake of sodium (WHO: 2 g / day; AHA: 1,5 g / day).
The actual daily sodium intake is often above these values. The reason for this is our relatively high salt consumption (2,5 g salt contains approx. 1 g sodium). The National Consumption Study II (NVS II) of the Max Rubner Institute, in which sodium consumption was determined using questionnaires, showed a median intake of 3,2 g / day (men) and 2,4 g / day (women) . The actual sodium intake is presumably even higher, as the recording via questionnaires is prone to errors. The determination of sodium in 24-hour urine serves as the gold standard for determining sodium intake. According to a report by the WHO, the sodium excretion in the INTERSALT study in various places in Germany was 4,1–4,5 g / day (men) and 2,7–3,5 g / day (women).
Regulation of the sodium balance
The sodium content is strictly controlled and is closely related to the regulation of the water balance. The normal concentration of sodium in serum is around 135–145 mmol / l. If the sodium level is lower, it is referred to as hyponatremia, in which there is an increase in cell volume. In hypernatremia, on the other hand, the sodium level is too high and the cells shrink. In both cases, the main impairment is the functioning of the brain. It can lead to epileptic seizures and disturbances of consciousness up to a coma. The renin-angiotensin-aldosterone system, adiuretin and atriopeptin play an important role in regulation.
The key organ in the regulation of sodium is the kidney. This is responsible for holding back water in the event of an excess of sodium in order to dilute the sodium in the body and to excrete sodium itself. If there is a sodium deficiency, more water is excreted and sodium is retained. It should be noted, however, that the kidneys need some time before they can react to the changed sodium level.
Distribution in the cells
The Na + ions are not evenly distributed in the organism; rather, as with the other ions, the concentrations inside and outside the cells are very different. These concentration gradients of Na + - and Cl− (mainly outside), K + - and organic anions (mainly inside) determine the majority of the membrane potential of living cells. This membrane potential and the ion gradients are vital for most cells. Since the small inorganic ions constantly migrate to the neighboring area because of the differences in concentration, an active process is required to counteract this. The most important role is played by the sodium-potassium pump, which repeatedly pumps Na + and K + ions back while consuming energy.
Functions in nerve cells
Na + ions play an important role in the generation and transmission of excitations in nerve cells (and muscle fibers). At the postsynapses of nerve cells (and on the neuromuscular endplate of the muscle fibers) there are certain receptors that, after being activated by neurotransmitters released by the preceding nerve cell when it is excited, open and become permeable to sodium ions. The influx of sodium causes a local change in the cell's membrane potential, which is stable in the ground state. The inside becomes less negative compared to the outside, this is called a depolarization. If this depolarization is still strong enough on the way to the axon, another type of sodium channel opens. These are the voltage-dependent sodium channels of the axon, which transmit the local depolarization - together with other ion channels - through a specific opening and closing rhythm. A continuous voltage wave, the action potential, is created on the axons of the nerve cells. The sodium-potassium pump plays an essential role in restoring the basic state.
Sodium in plants
In plants, however, sodium plays a subordinate role. While potassium is essential for all plants and most microorganisms, sodium is only required by some C4 and CAM plants, but usually not by C3 plants. Depending on the location, however, plants that can benefit from sodium intake have developed independently of this. These plants, called halophytes, are particularly common in coastal regions or other areas where the soil has a high concentration of sodium. Halophytes such as sugar beet, cabbage and many C4 grasses are salt-tolerant, as they can transport the sodium out of the central cylinder into the vacuoles of the leaf cells, where, as an osmotically effective ion, it increases the turgor and thereby increases the cell elongation and instead of potassium positively influences leaf area growth. Sodium thus partially replaces potassium, but in another part it also has an additional growth-promoting effect.
Plants that cannot transport sodium from the central cylinder into the leaf cells accumulate it in the xylem parenchyma. These so-called natrophobic plants include beans and maize, among others. The sodium, if it got into the leaf cells, could not be transported into the vacuoles, but would remain in the cell plasma (cytosol) and there would displace the potassium that is important for the formation of polymers (sodium-induced potassium deficiency). This ultimately led to an inhibition of photosynthesis. The accumulation of sodium in the central cylinder of the root and in the stem tissue has a negative effect on the plant when the sodium concentration is high. The increase in the osmotic value prevents it from absorbing and transporting water. The leaves are insufficiently supplied with water and nutrients, which leads to a reduction in photosynthesis.
Since most plants only contain small amounts of sodium, many herbivores must ingest additional sodium chloride from natural salt deposits.
safety instructions
Smaller amounts of sodium are stored under petroleum. For larger quantities there are integrated handling systems with a protective gas atmosphere. Despite protective gas or petroleum, the sodium is often covered by a layer of sodium hydroxide and sodium oxide.
Sodium fires can be extinguished with metal fire powder (table salt), potassium chloride, gray cast iron shavings, or as a makeshift with sand or dry cement. However, sand and cement react to a certain extent with sodium, which reduces the extinguishing effect. Under no circumstances may water, foam, dry powder, carbon dioxide or halons be used. Some of these extinguishing agents react strongly exothermically with sodium, which can lead to more severe fires and explosions.
Connections
In compounds, sodium occurs exclusively in the +1 oxidation state. All compounds have a strongly ionic character, and almost all of them are readily soluble in water. Sodium compounds are among the most important salts of many acids. Sodium salts are mostly used industrially to obtain the corresponding anions, as their synthesis is inexpensive.
halogen compounds
Sodium chloride (NaCl), often referred to as table salt or table salt, is the most important and best-known sodium salt. Since it occurs in large quantities, it is the most important raw material for the extraction of sodium and other sodium compounds. Sodium chloride is the most important source of sodium for humans. Technically, it is used, among other things, to preserve food and as road salt in road traffic. It is named after the sodium chloride structure, a crystal structure typical of many salts.
In addition, all other possible sodium halides, i.e. sodium fluoride NaF, sodium bromide NaBr and sodium iodide NaI, are known and stable.
oxygen compounds
A total of five oxides of sodium are known. These are sodium oxide Na2O, sodium peroxide Na2O2, sodium hyperoxide NaO2, disodium trioxide Na2O3 and sodium trioxide NaO3. Sodium oxide is contained in many glasses; it is created from the sodium carbonate used in glass production. When sodium is burned, it is only produced at certain temperatures (150–200 ° C) and stoichiometrically used amounts of sodium and oxygen. If this is not the case, sodium burns to sodium peroxide. This is a strong oxidizing agent and technically the most important sodium oxide. It is used as a bleach for textiles and paper, as well as a source of oxygen in diving and in submarines. The other oxides are very unstable and decompose quickly.
Sodium hydroxide (NaOH) is one of the most important bases in industry. The aqueous solution of sodium hydroxide is called caustic soda. It is used, among other things, for the production of soap and dyes as well as for the digestion of bauxite in aluminum production.
sulfur compounds
Sodium forms two salts with hydrogen sulphide, sodium sulphide Na2S and sodium hydrogen sulphide NaHS. Both are used, among other things, for heavy metal precipitation.
Sodium sulphate Na2SO4, the sodium salt of sulfuric acid, is used in detergents and in the paper industry in the sulphate process. Like other divalent anions, sulphate also forms sodium hydrogen sulphate in addition to sodium sulphate. Other oxy-sulfur acids also form sodium salts. An example is sodium thiosulphate Na2S2O3, which is used as a fixing salt in analog photography.
hydrides
In sodium hydride NaH and sodium borohydride NaBH4, the hydrogen is in the −1 oxidation state. Both are primarily used in organic chemistry. Sodium hydride is essentially used as a strong, little nucleophilic base for the deprotonation of thiols, alcohols, amides, CH-acidic compounds, etc., while sodium borohydride is used to reduce z. B. of ketones. The latter reaction can be carried out selectively for ketones by the presence of cerium (III) compounds (Luche reduction). If they come into contact with water, gaseous hydrogen H2 is produced.
More sodium compounds
Sodium carbonate Na2CO3 and sodium hydrogen carbonate NaHCO3 are the sodium salts of carbonic acid. Along with sodium chloride and sodium hydroxide, they are among the most important sodium compounds. Sodium carbonate (often referred to by the common name soda) is used in large quantities in glass production. Sodium hydrogen carbonate is used as baking soda. When heated, it forms carbon dioxide and water with acids.
Sodium nitrate NaNO3, the sodium salt of nitric acid, is one of the rare naturally occurring nitrate compounds (Chile's nitrate). Sodium nitrate is used as a fertilizer and as a preservative.
Organic compounds of sodium, in contrast to those of lithium, are very unstable. They are extremely reactive and can sometimes react with otherwise unreactive aliphatic hydrocarbons. Only compounds with aromatic radicals, such as cyclopentadiene, which can be used as reducing agents, are sufficiently stable for applications in reactions.
Soaps are sodium or potassium salts of fatty acids. For production, fats are boiled with a caustic soda or potassium hydroxide solution. This process is called soap boiling, the chemical reaction saponification. The fats are broken down into glycerine and the alkali salts of the fatty acids (the actual soaps). Alternatively, soaps can be made directly from free fatty acids by reacting them with alkalis to form their salts. Suitable fatty acids are, for example, lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and ricinoleic acid.






