Hafnium is a chemical element with the symbol Hf and the atomic number 72. It is named after the Latin name of the city of Copenhagen, Hafniain which the element was discovered. It is a silver-gray, shiny, corrosion-resistant transition metal that is in the 4th subgroup (group 4) or titanium group in the periodic table.
Hafnium has very similar properties to the zirconium directly above it in the periodic table. Biological functions are not known, it does not normally occur in the human organism and it is not toxic.
Hafnium was one of the last stable elements on the periodic table to be discovered. The first indication of the existence of another element between lutetium and tantalum arose from Moseley's law found in 1912. In 1914, Henry Moseley attempted to find the unknown but expected element with the atomic number 72 according to this law in samples of rare earth minerals (nowadays lanthanides). But he was unsuccessful.
In his work on atomic theory published in 1922, Niels Bohr predicted that the lanthanide series with lutetium would come to an end and that element 72 must therefore be similar to zirconium. Hafnium could be detected just one year later: In 1923, Dirk Coster and George de Hevesy discovered it in Copenhagen using X-ray spectroscopy in Norwegian zirconium. Further investigation of other minerals showed that hafnium is always contained in zirconium-containing minerals. Jantzen and Hevesy succeeded in separating them from zirconium by repeatedly crystallizing the diammonium and dipotassium fluorides of the two elements. Elemental hafnium could then be obtained by reduction with sodium.
occurrence
Hafnium, with a content of 4,9 ppm in the continental crust, is an element that is not very common on earth. In terms of frequency, it is comparable to the elements bromine and cesium and more common than the long-known gold and mercury. Hafnium does not occur naturally or in its own minerals. Zirconium minerals such as zircon and baddeleyite, on the other hand, always contain hafnium; the amount of hafnium is usually 2% of the zirconium content (1-5 percent by weight of hafnium). One of the few minerals that contain more hafnium than zirconium is the zircon variety Alvit [(Hf, Th, Zr) SiO4].
Analogous to zirconium, the most important hafnium deposits are the zirconium deposits in Australia and South Africa. The reserves are estimated at 1,1 million tons (calculated as hafnium oxide).
Extraction and presentation
In order to obtain hafnium, it has to be separated from the zirconium. This is not possible during the manufacturing process, but takes place in a separate process. Extraction processes are used for the separation. The different solubility of certain zirconium and hafnium salts in special solvents is used. Examples of this are the different solubilities of nitrates in tri-n-butyl phosphate and that of thiocyanates in methyl isobutyl ketone. Other possible separation options are ion exchangers and the fractional distillation of suitable compounds.
After the Kroll process, the separated hafnium can first be converted into hafnium (IV) chloride and then reduced to elemental hafnium with sodium or magnesium.
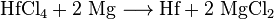
If even purer hafnium is required, the Van Arkel de Boer process can be used. During the heating under vacuum, the hafnium initially reacts with iodine to form hafnium (IV) iodide. This is broken down again into hafnium and iodine on a hot wire.

Hafnium is only produced in small quantities on a scale of 100 tons. It is not produced in-house, but is a by-product of the extraction of hafnium-free zirconium for fuel rods.
Features
Physical Properties

Crystal structure of α-Hf, a = 320 pm,c = 505 pm
Hafnium is a silvery, shiny heavy metal of high density (13,31 g / cm3). It crystallizes in two different modifications depending on the temperature. Under normal conditions it crystallizes in a hexagonal close packing of spheres (α-Hf) and is therefore isotypic to α-Zr, above 1775 ° C it changes into a body-centered cubic structure (β-Hf).
If the purity of hafnium is high, it is relatively soft and pliable. It is easy to work by rolling, forging and hammering. If, on the other hand, there are traces of oxygen, nitrogen or carbon in the material, it becomes brittle and difficult to process. The melting and boiling points of hafnium are the highest in the group at 2227 ° C and 4450 ° C (melting point: titanium: 1667 ° C, zirconium: 1857 ° C).
In almost all other properties, the metal resembles its lighter homologue zirconium. This is caused by the lanthanide contraction, which causes similar atomic and ionic radii (atomic radii Zr: 159 pm, Hf: 156 pm). An exception is the density of the zirconium with 6,5 g / cm3 has a significantly lower value. A technically important difference is that hafnium can absorb neutrons 600 times better. This is the reason why the hafnium has to be separated for the use of zirconium in nuclear power plants.
Hafnium is superconducting below the transition temperature of 0,08 K.
Chemical properties
Hafnium is a base metal that reacts with oxygen to form hafnium dioxide when heated. Other non-metals, such as nitrogen, carbon, boron and silicon, also form compounds under these conditions. A dense oxide layer quickly forms at room temperature, which passivates the metal and protects it from further oxidation.
Hafnium is stable in most acids because of its passivation under normal conditions. It corrodes quickly in hydrofluoric acid; noticeable corrosion occurs in hot, concentrated sulfuric and phosphoric acid. Hydrochloric acid-nitric acid mixtures, including aqua regia, should only be exposed to hafnium for a short time, even at room temperature; at 35 ° C, removal rates of more than 3 mm / year must be expected. In aqueous bases it is resistant up to a temperature of approx. 100 ° C, the material removal is usually less than 0,1 mm / year.
isotope
There are a total of 35 isotopes and 18 nuclear isomers of hafnium 153Hf to 188Hf known. Natural hafnium is a mixed element that consists of a total of six different isotopes. The most common isotope is with a frequency of 35,08% 180Hf. It follows 178Hf with 27,28%, 177Hf with 18,61%, 179Hf with 13,62%, 176Hf with 5,27% and 174Hf at 0,16%. The only natural isotope is 174Hf weakly radioactive, it is an alpha emitter with a half-life of 2 x 1015 Years. The isotopes 177Hf and 179Hf can be detected with the aid of NMR spectroscopy.
The core isomer 178 2mWith a half-life of 31 years, Hf is long-lived and at the same time emits strong gamma radiation of 2,45 MeV when it decays. This is the highest energy that an isotope that is stable over a long period of time emits. One possible application is to use this core isomer as a source in powerful lasers. In 1999, Carl Collins discovered that the isomer could release its energy in one fell swoop when exposed to X-rays. However, possible applications, such as explosives, are unlikely.
Usage
Hafnium sheet from industrial waste
Because it is difficult to extract, hafnium is only used in small quantities. The main area of application is nuclear technology, in which hafnium is used as a control rod to regulate the chain reaction in nuclear reactors. The use of hafnium has several advantages over other possible neutron absorbing substances. The element is very resistant to corrosion and the nuclear reaction with the neutrons creates hafnium isotopes, which also have high absorption cross-sections. Due to the high price, it is often only suitable for military applications, for example for reactors in atomic submarines.
There are a few other uses. Hafnium reacts quickly with small amounts of oxygen and nitrogen and can therefore be used as a getter substance to remove the smallest amounts of these substances from ultra-high vacuum systems. When burned, the metal emits a very bright light. It is therefore possible to use hafnium in flash lamps with a particularly high luminous efficiency. Several very stable and high-melting compounds, especially hafnium nitride and hafnium carbide, can be made from the elements.
In alloys with metals such as niobium, tantalum, molybdenum and tungsten, an addition of 2% hafnium increases the strength. Particularly stable, high-melting and heat-resistant materials are created.
safety instructions
Like many other metals, hafnium is highly flammable and pyrophoric in its finely divided state. In contrast, it is not flammable when compact. The metal is not toxic. For these reasons, no special safety regulations need to be observed when handling hafnium.
Connections
Hafnium forms a number of compounds. These are mostly salts or mixed crystals and often have high melting points. The most important oxidation state of hafnium is + IV, but compounds in lower oxidation states, from 0 to + III, and in complexes also negative oxidation states are known.
Hafnium (IV) oxide
Hafnium (IV) oxide is a very stable and high-melting solid. It has a high relative permittivity of 25 (for comparison: silicon dioxide: 3,9). It can therefore be used as a high-k dielectric to isolate the control connection (gate) for microprocessors. By further reducing the structure widths, leakage currents are becoming an ever greater problem, because the miniaturization of CMOS structures also requires thinner gate insulation. The undesired leakage current increases sharply below 2 nm due to the tunnel effect. By using a high-k dielectric, the thickness of the dielectric can be increased again in order to reduce leakage current without the transistor having a loss in performance (reduction in switching speed). Thus, thicker dielectrics allow further miniaturization.
Other hafnium compounds
Hafnium carbide is one of the substances with the highest melting points. Together with hafnium nitride and hafnium boride, it belongs to the hard materials.
There are some known halogen compounds of hafnium. In the oxidation state + IV exist both the fluoride and chloride, bromide and iodide. Hafnium (IV) chloride and hafnium (IV) iodide play a role in the production of hafnium. In the lower oxidation states only chlorine and bromine compounds, and hafnium (III) iodide are known.
The potassium hexafluoridohafnate (IV) K2[HfF6] as well as the ammonium hexafluoridohafnate (IV) (NH4)2[HfF6] can be used to separate the hafnium from zirconium, as both salts are more soluble than the corresponding zirconium complexes.
| General | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, atomic number | Hafnium, Hf, 72 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 4, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | steel gray | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-58-6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth shell | 4,2 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||
| atomic mass | 178,49 u | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 155 (208) pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 150 pm | ||||||||||||||||||||||||||||||||||||||||||||||||
| electron configuration | [Xe] 4f14 5d2 6s2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| 1. ionization | 658,5 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 2. ionization | 1440 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 3. ionization | 2250 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| 4. ionization | 3216 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | fixed | ||||||||||||||||||||||||||||||||||||||||||||||||
| modifications | two (α- / β-Hf) | ||||||||||||||||||||||||||||||||||||||||||||||||
| crystal structure | hexagonal | ||||||||||||||||||||||||||||||||||||||||||||||||
| density | 13,28 g / cm3 (25 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 5,5 | ||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( = 7,0 10−5) = 7,0 10−5) |
||||||||||||||||||||||||||||||||||||||||||||||||
| melting point | 2506 K (2233 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 4876 K (4603 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 13,44 · 10−6 m3/ mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 630 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| heat of fusion | 25,5 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| vapor pressure | 0,00013 Pa at 1970 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| speed of sound | 3010 m / s at 293,15 K | ||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 140 J / (kg · K) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 3,12 · 106 A / (V · m) | ||||||||||||||||||||||||||||||||||||||||||||||||
| thermal conductivity | 23 W / (m K) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical | |||||||||||||||||||||||||||||||||||||||||||||||||
| oxidation states | 4 | ||||||||||||||||||||||||||||||||||||||||||||||||
| normal potential | −1,505 V (HfO2 + 4 H.+ + 4 e- → Hf + 2 H2O) |
||||||||||||||||||||||||||||||||||||||||||||||||
| electronegativity | 1,3 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||
| isotope | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||



