REE as a by-product of bauxite mining
Rare earths (REE) in Al and Fe (oxy) hydroxides in bauxites of Provence and Languedoc (southern France): impact on the potential recovery of rare earths as by-products of bauxite mining
Summary:
Bauxite in southern France (Provence and Languedoc) have been used since the beginning of the last century. Although most deposits today are sub-economic or degraded, these bauxites represent model analogues for other economic bauxites in the world. These chalky karstic deposits lie directly on Jurassic Carbonates and have emerged through a combination of different processes: in situ alteration of siliceous sediments occurring on carbonate platforms and post-processing of early bauxites in the karstic network. In this study we present preliminary geochemical and in-situ laser ablation (LA) of bulk rocks -ICP-MS analyzes on Al- and Fe-Oxy-hydroxides of Provence (Les Baux-de-Provence) and Languedoc (Villeveyrac, Loupian) Bauxite, with the aim to evaluate the concentrations of rare earth elements (REEs) and their deportation in these minerals. REEs have an overall average concentration of 700 mg / kg in the analyzed samples, which are predominantly boehmite, γ-AlO (OH), and Fe-oxyhydroxides (hematite and goethite). Maximum REE concentrations are often associated with positive Ce anomalies in chondrite normalized patterns. In contrast to other examples from the literature, it has been observed that high REE concentrations also occur in samples that are apparently free or low in REE minerals. In these samples, the total amount of REEs is positively correlated with that of Ga (often contained in boehmite). LA-ICP-MS Trace element analyzes on boehmite and Fe-oxy-hydroxides have shown that although the Al hydroxide contains the suite of REEs, goethite and hematite are preferably only enriched in Ce. Since Al-hydroxides are digested during the Bayer process, it is an interesting topic to develop in the future whether (and how) the rare earths released in the Al-hydroxide digestion can be recovered together with Al from the pregnant liquor, as this is common at Ga.
1. Introduction
Bauxite occurrences are economic concentrations of aluminum that are residual products of weathering and leaching of aluminosilicate base rocks. These deposits typically occur in humid, tropical to subtropical climates with annual rainfall greater than 1,2 m and annual average temperatures greater than 22 ° C [1]. Bauxites can be subdivided into lateritic bauxites, which are formed in lateritic soil profiles, and karstic soils, which occur instead on carbonate rocks [2]. Aluminum in bauxites occurs mainly in the form of gibbsite [Al (OH) 3] or amorphous aluminum hydroxides. Boehmite [γ-AlO (OH)] and diaspore [α-AlO (OH)] (both less hydrated than gibbsite) typically occur in bauxites exposed to multiple diagenetic stages and / or metamorphoses, or in metabauxites [3]. Iron is separated from the aluminum and is generally concentrated as hematite and subordinate to goethite. Anatase (TiO2), kaolinite [Al2Si2O5 (OH) 4] and other clays may also be present in bauxites in various proportions [3].
World bauxite resources are estimated to be 55-75 billion tonnes occurring in Africa (32%), Oceania (23%), South America and the Caribbean (21%), Asia (18%), and elsewhere (6%) [4 ]. Approximately 70-80% of global bauxite production is first processed to aluminum oxide (Bayer process [5]) and then to aluminum by smelting and electrolysis (Hall-Hérault process). The modern version of the Bayer process retains the essential steps of dissolving alumina-rich minerals in a hot liquor and separating the insoluble phases, followed by alumina precipitation and calcination to alumina (Al2O3) [5]. The main advantage of the Bayer process is that the soda solution treatment specifically affects aluminum-containing minerals, thus minimizing the dissolution of other mineral phases [5]. This digestion is carried out in a strongly alkaline solution at temperatures above 100 ° C (the reaction for boehmite-rich bauxite is greater than 240 ° C), so that minerals such as gibbsite and boehmite are dissolved to form (Al (OH) 4) - (aluminate ) Ions in solution [5]. The insoluble phases of the bauxite (ie the residue, called red mud or sludge) are separated from the main liquor and washed to recover soda and aluminum. Aluminum in solution in the liquid is precipitated to Al (OH) 3 and / or AlOOH, which are finally calcined to alumina (Al2O3) [5].
Recently, great attention has been given to the processes of controlling the distribution and possible extraction techniques of several smaller elements in bauxite deposits, especially those (eg rare earth REEs, Ga, Sc) which, due to their high economic importance and their high supply risk, are at international level Markets are considered to be critical for Western countries and the European Union [6,7,8,9,10,11,12,13,14,15,16,17,18,19]. As a byproduct of bauxite treatment, currently only gallium is recovered that occurs at average concentrations of 50 mg / kg [20] since it is released during the digestion phase of the Bayer process from gibbsite, boehmite and diaspore [21]. There is an ongoing debate on the consideration of bauxite deposits as potential resources of rare earths [4,22,23,24,25,26,27,28]. Several studies focused mainly on the occurrence of REE-containing minerals in red mud and on REE mobility during the Bayer process. In particular, Vind et al. [27] have shown that during the Bayer process, while Ga, V, As, and Cr are mainly accumulated in process fluids, most other trace elements (REE and Sc) tend to be unaffected in solid phases stay and get completely into the Bauxitrest. Vind et al. [27] also demonstrated that the most abundant phase containing REEs in the remainder of Greek bauxites is an LREE ferrotitanate [(REE, Ca, Na) (Ti, Fe) O3] compound. which is said to have formed during the digestion of precursor LREE minerals during the Bayer process. Minor amounts of LREEs appeared as carbonates and phosphates, while heavy REEs were found in connection with a Y-phosphate phase [27].
In this study, we present geochemical and in situ laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS) analyzes of Al and Fe oxyhydroxides in bauxite deposits in southern France with the aim of detecting REE concentrations and deportation in to evaluate these minerals. We have taken samples from old bauxite mines in Provence (Les Baux-de-Provence) and Languedoc (Villeveyrac, Loupian) (Figure 1). Today, France has a small bauxite market, but this country had a historical total bauxite production of about 1885 million tons from 1991 to 101. 1972, together with Hungary and Greece, supplied more than 11% of world bauxite production (http://sigminesfrance.brgm.fr/) to France. The bauxite deposits of the south of France are karstic bauxites associated with stratigraphic fractures in the Jurassic-Cretaceous carbonate sequences [29,30,31,32]. They originated between the Albanian and the early Cenomanian through a combination of different processes: in situ alteration of siliceous sediments deposited on formed carbonate platforms, and post-processing of early bauxite [30,31,32] bauxites. Silicate sediments were formed by the erosion of the exposed Hercynian cellar (eg Massif Central, Maures Massif) near the carbonate platforms [30,31,32]. These deposits mainly contain boehmite-gibbsite mineralization [30,31,32] and may be considered model analogs for economic karstic construction in other regions of the world.
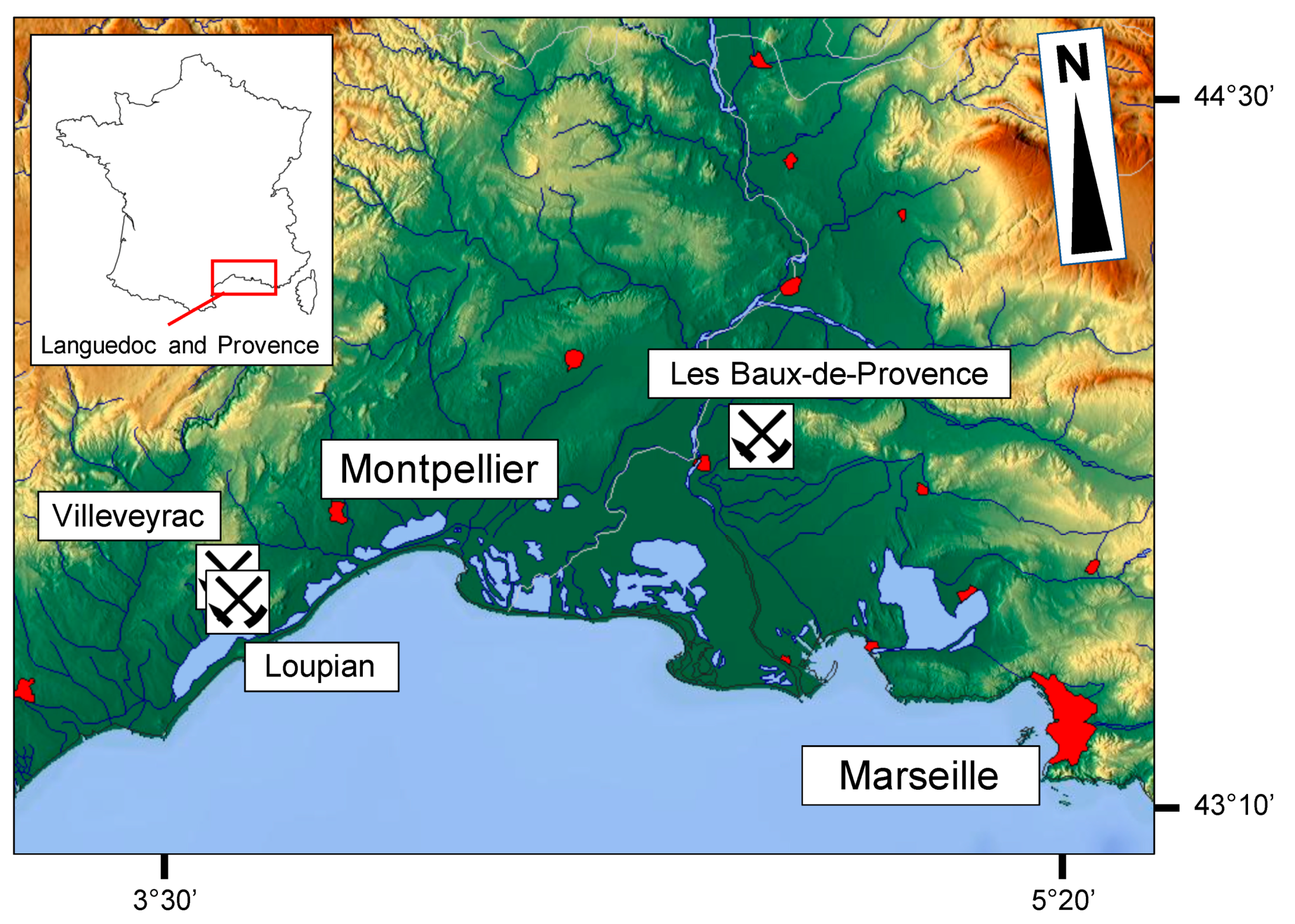
Figure 1. Relief map of Provence and Languedoc (southern France), with the approximate locations of the old bauxite mines. Object of sampling (https://maps-for-free.com).
2. Materials and methods
Studies were carried out on a sample from Provence (Les Baux-de-Provence) and three from Languedoc (Villeveyrac, Loupian) bauxite deposits (Figure 2, Table 1). Two samples of Loupian were collected at the top and bottom of the bauxite profile. The samples were divided into halves: the first half was used to produce polished blocks for optical and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS), while the second half was used to prepare powder for whole-rock X-ray powder diffraction (XRPD) and chemical analysis has been. The blocks were mounted in two-component epoxy resin (SpeciFix20), polished with diamond suspension (1 μm) and aluminum oxide Al2O3 (0,3 μm) and polished before the REM
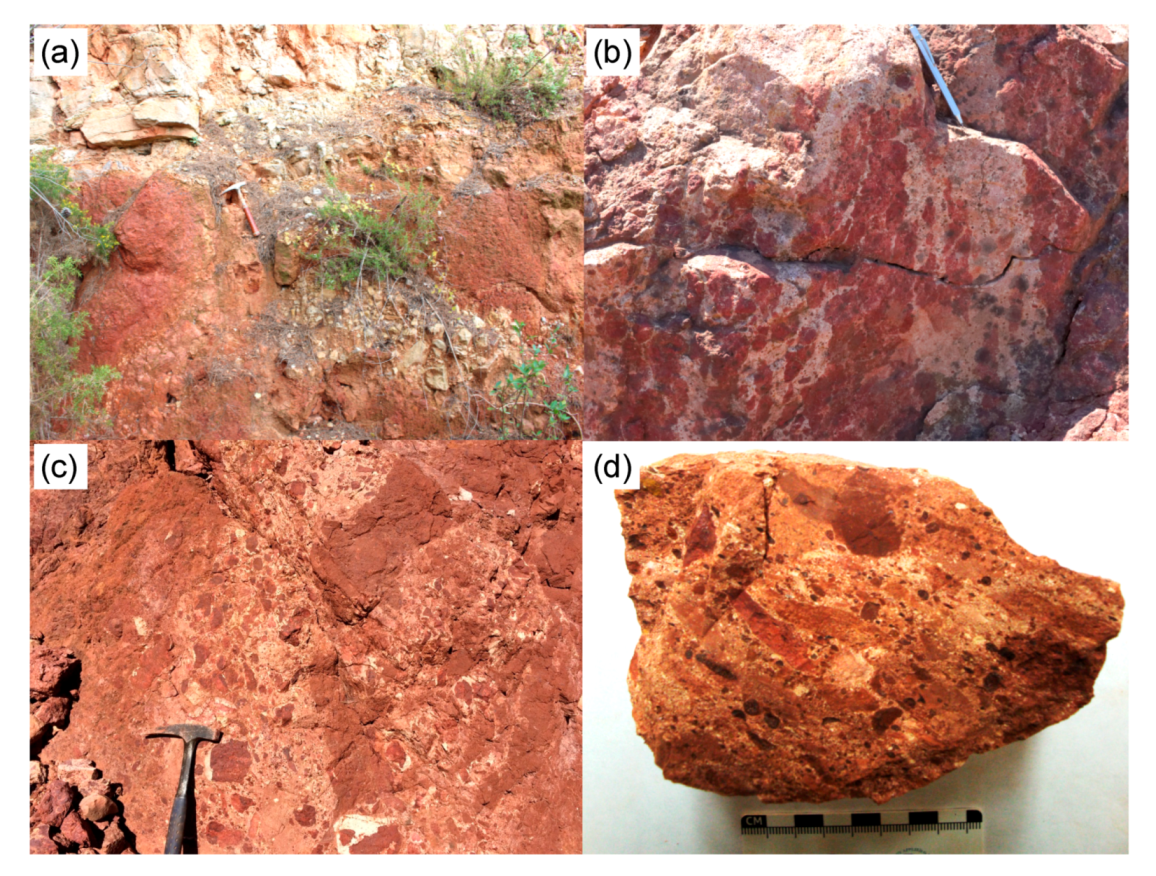
Figure 2. Outcrops and samples of localities considered in this study: (a) Les Baux-de-Provence: upper part of the bauxite profile, (b) Villeveyrac: bleached structure in the bauxite horizon, (c) Loupian: detrital features in the decomposition area, (d) Loupian : Sample LUP_A15.
Table 1. (a) Locations, (b) mineralogy, and (c) major, minor, and trace element compounds of the bauxite samples tested.
| (a) | ||||||
| Locations | LBP 7 | Vil IV-5 | LUP_A4 | LUP_A15 | ||
| Latitude | 43 ° 44'13 "N | 43 ° 30'16 "N | 43 ° 27'27 "N | 43 ° 27'26 "N | ||
| Longitude | 4 ° 46'20 "E | 3 ° 37'52 "E | 3 ° 38'01 "E | 3 ° 38'01 "E | ||
| (B) | ||||||
| Mineral | LBP 7 | Vil IV-5 | LUP_A4 | LUP_A15 | ||
| Min-Max (wt.%) | ||||||
| kaolinite | 16 – 17 Feet | 39 – 40 Feet | 4 – 5.5 Feet | 4 – 5 Feet | ||
| boehmite | 53 – 58 Feet | 22 – 23 Feet | 63 – 68 Feet | 76 – 77 Feet | ||
| goethite | 4 – 10 Feet | 10 – 11 Feet | 10 – 17 Feet | 0.5 – 1 Feet | ||
| hematite | 15 – 22 Feet | 22 – 27 Feet | 10 – 20 Feet | 12 – 15 Feet | ||
| anatase | 2 – 2.3 Feet | 1.7 – 1.9 Feet | 3.1 – 3.7 Feet | 2.5 – 3.4 Feet | ||
| calcite | - | 3 | - | - | ||
| (C) | ||||||
| Method | Analyte | Det. Lim. | LBP 7 | Vil IV-5 | LUP_A4 | LUP_A15 |
| wt.% | ||||||
| XF701 | SiO2 | 0.01 | 8.14 | 18.30 | 2.53 | 2.28 |
| XF701 | Al2O3 | 0.01 | 51.90 | 34.30 | 55.00 | 66.40 |
| XF701 | TiO2 | 0.01 | 2.32 | 1.85 | 3.74 | 3.39 |
| XF701 | Fe2O3 | 0.01 | 24.10 | 32.50 | 24.90 | 13.30 |
| XF701 | Cr2O3 | 0.001 | 0.07 | 0.04 | 0.05 | 0.04 |
| XF701 | Dog | 0.01 | 0.10 | 1.67 | 0.08 | 0.26 |
| XF701 | MgO | 0.01 | 0.07 | 0.12 | 0.14 | 0.08 |
| XF701 | MnO | 0.01 | 0.03 | 0.02 | 0.05 | 0.05 |
| XF701 | Na2O | 0.01 | 0.04 | 0.06 | 0.04 | 0.04 |
| XF701 | K2O | 0.01 | <0.01 | 0.06 | <0.01 | <0.01 |
| XF701 | P2O5 | 0.001 | 0.14 | 0.24 | 0.16 | 0.10 |
| XF701 | V2O5 | 0.001 | 0.10 | 0.09 | 0.09 | 0.07 |
| XF701 | ZrO2 | 0.01 | 0.08 | 0.08 | 0.09 | 0.09 |
| XF701 | SO3 | 0.01 | 0.03 | 0.01 | 0.02 | 0.03 |
| XF701 | LOI | 12.40 | 10.60 | 12.80 | 13.30 | |
| XF701 | 0.01 | 99.51 | 99.93 | 99.69 | 99.44 | |
| mg / kg | ||||||
| LF100 | Ba | 1 | 14 | 26 | 11 | 11 |
| LF100 | Be | 1 | 2 | 5 | 9 | 5 |
| LF100 | Co | 0.2 | 14.8 | 18.8 | 37.1 | 18.1 |
| LF100 | Cs | 0.1 | <0.1 | 0.5 | <0.1 | <0.1 |
| LF100 | Ga | 0.5 | 66.4 | 38.3 | 66.9 | 70.5 |
| LF100 | Hf | 0.1 | 21.2 | 19.8 | 25.8 | 28.3 |
| LF100 | Nb | 0.1 | 51.9 | 40.3 | 78.4 | 74.2 |
| LF100 | Rb | 0.1 | 0.4 | 2.3 | <0.1 | <0.1 |
| LF100 | Sn | 1 | 12 | 9 | 15 | 15 |
| LF100 | Sr | 0.5 | 122.4 | 379.8 | 196 | 72.8 |
| LF100 | Ta | 0.1 | 3.7 | 2.9 | 5.4 | 4.9 |
| LF100 | Th | 0.2 | 51 | 35.3 | 45.6 | 49.4 |
| LF100 | U | 0.1 | 13.3 | 7.6 | 17.4 | 16 |
| LF100 | V | 8 | 538 | 484 | 469 | 377 |
| LF100 | W | 0.5 | 6.9 | 5.6 | 8.6 | 9.7 |
| LF100 | Zr | 0.1 | 773.0 | 733.1 | 991.3 | 1117.4 |
| LF100 | Y | 0.1 | 52.0 | 84.6 | 85.4 | 62.8 |
| LF100 | La | 0.1 | 230.3 | 147.5 | 117.5 | 81.1 |
| LF100 | Ce | 0.1 | 401.1 | 326.2 | 383.8 | 116 |
| LF100 | Pr | 0.02 | 49.69 | 29.66 | 19.95 | 10.80 |
| LF100 | Nd | 0.3 | 178.2 | 105.1 | 64.7 | 28.7 |
| LF100 | Sm | 0.05 | 30.63 | 20.04 | 11.64 | 5.10 |
| LF100 | Eu | 0.02 | 4.96 | 4.08 | 2.48 | 1.18 |
| LF100 | Gd | 0.05 | 16.06 | 18.49 | 12.18 | 5.98 |
| LF100 | Tb | 0.01 | 1.88 | 2.79 | 2.17 | 1.33 |
| LF100 | Dy | 0.05 | 10.21 | 15.79 | 14.19 | 9.54 |
| LF100 | Ho | 0.02 | 1.88 | 3.08 | 3.03 | 2.22 |
| LF100 | Er | 0.03 | 5.64 | 9.06 | 9.43 | 7.12 |
| LF100 | Tm | 0.01 | 0.90 | 1.35 | 1.42 | 1.11 |
| LF100 | Yb | 0.05 | 6.37 | 8.58 | 9.64 | 7.49 |
| LF100 | Lu | 0.01 | 0.96 | 1.33 | 1.51 | 1.19 |
| Σ REE + Y | 990.78 | 777.65 | 739.04 | 341.66 | ||
| Eu / Eu *(CHO) | 0.68 | 0.65 | 0.64 | 0.65 | ||
| (La / Yb) N(CHO) | 25.93 | 12.33 | 8.74 | 7.77 | ||
| Ce / Ce *(CHO) | 0.92 | 1.21 | 1.94 | 0.96 | ||
Qualitative XRPD analyzes were performed with a Seifert-GE ID3003 diffractometer, with CuKα radiation, Ni-filtered at 40 kV and 30 mA, 3-80 ° 2θ range, step scan 0,02 °, time 10 s / step at the dipartimento di Scienze della Terra, dell'Ambiente e delle Risorse (DiSTAR) University of Naples Federico II (Italy). The raw data were processed with the RayfleX (GE) software package. Semi-quantitative XRPD analyzes were carried out with the X'Pert PRO diffractometer from PANalytical, at the Istituto Nazionale di Geofisica e Vulcanologia-Osservatorio Vesuviano (Napoli), with a high-speed PIXcel detector, Ni-filtered, CuKα radiation, pyrolytic graphite crystal -Monochromator, performed at 40 kV and 40 mA in a range of 3-70 ° 2θ with 0,02 ° steps at 8 s / step. The interpretation of the diffraction pattern was done with the HighScore Plus software and the JCPDS PDF-2 database. The mineral abundances were determined based on the peak intensity ratio between the mineral phases and the total rock chemistry analyzes.
Full chemical analyzes of major and minor elements were carried out at Bureau Veritas Commodities Canada Ltd. carried out. (Vancouver, BC, Canada) on identical powder columns as used for XRPD analysis. Moisture and loss on ignition (LOI) were determined separately at 105 ° C and 1000 ° C. For the analysis of the main elements, dried samples were mixed with lithium tetraborate / metaborate flow, followed by fusion and casting in glass panes, then analyzed by X-ray fluorescence (XRF method XF701). Rare earths and refractory elements were determined by inductively coupled plasma mass spectrometry (ICP-MS) after LiBO2 / Li2B4O7 fusion (LF100 method, Bureau Veritas Commodities Canada Ltd.).
Scanning Electron Microscopy (SEM) EDS analyzes were performed with a ZEISS EVO LS 15 Scanning Electron Microscope (Natural History Museum, London, UK) at 20 kV, with 8,5 mm working distance and 3 nA current consumption with X-max detectors. A co-standard was used to calibrate the device.
Laser ablation (LA) ICP-MS analyzes were performed on an ASI NWR193 UV 193 nm short pulse width laser (<4 ns) equipped with a TwoVol2 ablation cell and coupled to an Agilent 7700x quadrupole ICP-MS, which is connected to two external Rotary Pumps is configured for increased sensitivity and is located in the LODE Laboratory-Natural History Museum (London). The ablation points were 35-50 µm in diameter, with a fluence of 3,5 J-cm-2, fired at a frequency of 10 Hz. The transport gas used was He mixed with at a flow rate of 0,5 L-min-1 Ar at a flow rate of 1,1 L-min-1, in a signal smoothing device. The item menus and the ICP-MS dwell time settings used to determine the composition of the various minerals are listed in the additional material (Table S1). The element ratios to an internal standard element (57Fe for both Fe oxyhydroxides and Al hydroxides) were determined by referring to background corrected integrated intensities from mineral signals on the external calibration standard. That was GSD-1g glass (USGS).
Absolute element concentrations were then calculated from internal standard element concentrations (given by SEM-EDS) in the program ExLAM [34]. The limits of detection were set on the conventional 3σ of the background signal variation [35]. NIST 2782, NIST 610 and BC_28 (the in-house magnetite standard of Dare et al. [36]) were also monitored during the Al-hydroxide and Fe-oxyhydroxide analysis. Contamination of the phase of interest by inclusion or exceeding of grain boundaries has been avoided by monitoring a number of non-formula elements associated with these contaminant phases. Time-resolved raw CP signals have been meticulously checked and the longest possible clean integration intervals (up to 60 s signal) maintained. Analyzes with significant contamination were discarded immediately. The tuning was optimized for the entire mass range, and the oxide formation (as represented by 248ThO / 232Th) and the formation of doubly charged species (observed over 22Ca / 44Ca) were kept below 0,2%.
3. Results
3.1. Mineralogy and geochemistry
The analyzed samples are represented by oolitic bauxites that have locally detrital features (Figure 2). The LBP 7 sample, collected at the bottom of the Baux-de-Provence (hereinafter Les Baux) Bauxithorizonts (Figure 2a), shows a matrix-assisted texture generated by centimetrical to millimeter-size bauxite lumps in a boehmitic, fe-boehmitic, and boehmitic-kaolinitic matrix is marked. The bauxite clasts consist predominantly of boehmite or hematite (Figure 3a). Boehmite-rich lumps usually have a simple concretionary or complex oolitic structure (Figure 3b). Kaolinite and anatase were found as single grains within the boehmitic matrix, which also contains detrital zircon, monazite and xeno-time. The LBP 7 sample has an Al2O3 concentration of 51,90% by weight, higher than the Fe2O3 and SiO2 concentrations (24,10% by weight and 8,14% by weight, respectively) (Table 1). The TiO2 concentration is 2,32% by weight. Among the trace elements there are significant concentrations of Ga (66 mg / kg), V (538 mg / kg) and Zr (773 mg / kg).
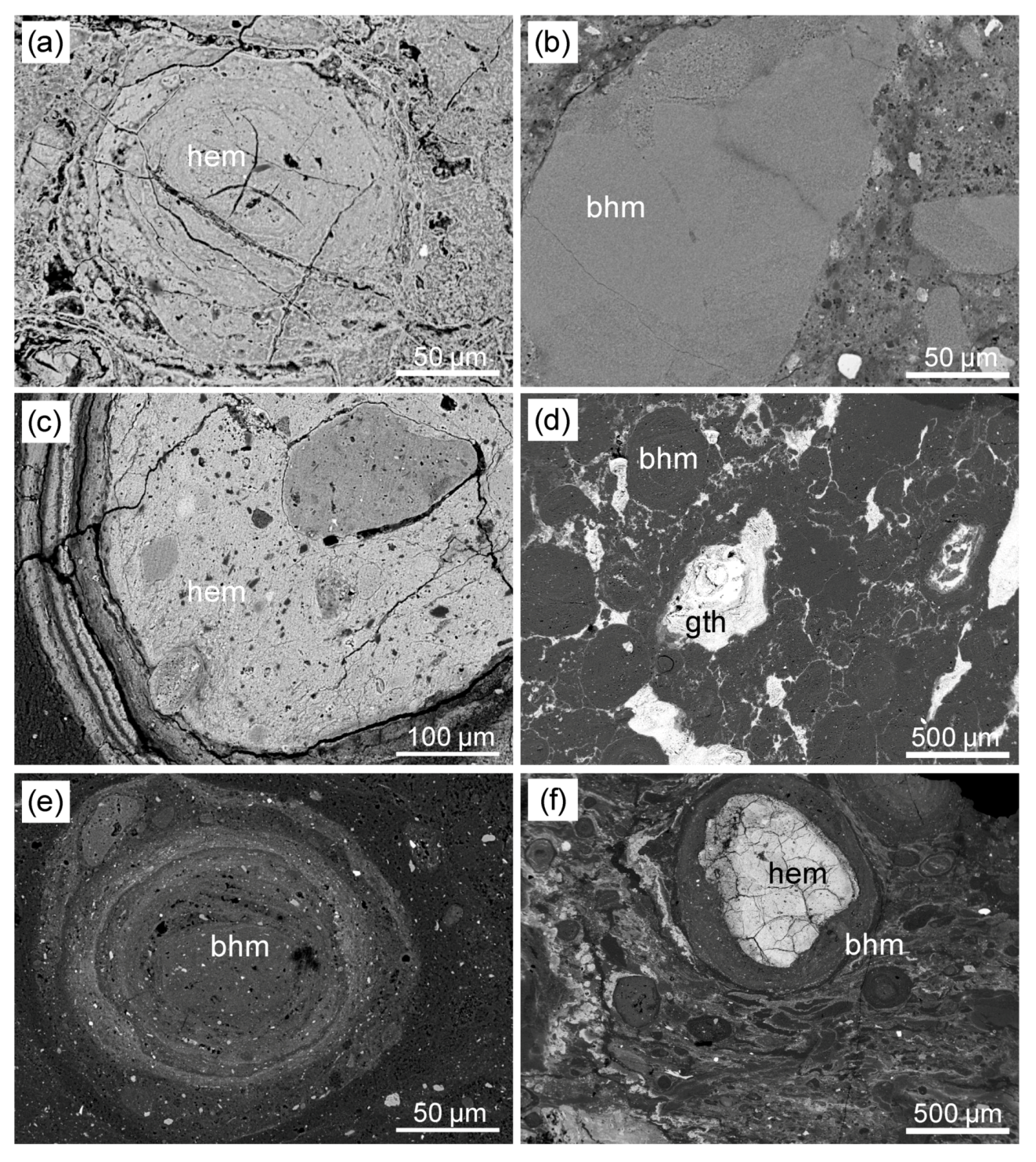
Figure 3. SEM (backscattered-electron) micrographs: (a) LBP 7: nucleus of a hematitic oolite, (b) LBP 7: boehmite-rich lumps, (c) Vil_IV5: complex hematitic oolite, (d) LUP_A4: goethite crusts and dendrites (light) between (a) LUP_A15: Boehmite Oolite, (f) LUP_A15: Oolite, which has a hematin core and a boehrite wall in a matrix of boehmitic micro-olives with compaction properties. bohm = boehmite, hem = hematite, gth = goethite.
The Vil_IV5 (Villeveyrac) sample has a matrix-based texture characterized by less than 20% modal on clasts larger than 2 mm, and contains abundant kaolinite (Table 1). The sample shows typical bleach strips (Figure 2b), which give the sample a banded structure. The bands alternately include boehmite to Fe boehmite rich zones associated with kaolinite, and are intersected locally by veins of pure boehmite. Interestingly, most of the oolites found in this sample consist of hematite (Figure 3c), while boehmite is concentrated in the sample matrix, which is finely mixed with kaolinite. Detrital minerals are mainly represented by zircon, rutile, monazite and xeno-time. This sample shows approximately similar concentrations of Al2O3 and Fe2O3 (34,30 wt% or 32,50 wt%), high SiO2 (18,30 wt%) and low TiO2 levels (1,85 wt%) (Table 1) , Gallium (38,3 mg / kg) and V (484 mg / kg) are not as high as in the LBP-7 sample, while Zr has a similar concentration (733 mg / kg). In this sample, the total amount of REE + Y is 777,65 mg / kg.
The two samples from the Loupian deposit were taken at the top (LUP_A4, Figure 2c) and at the bottom (LUP_A15, Figure 2d) at the bauxite horizon. In the LUP_A15 sample, the detrital features are more abundant than in the LUP_A4 sample. In particular, in the matrix of the LUP_A15 (bottom) sample, it is possible to observe millimeter to subcentimetric lumps of complex oolites or bauxite, while in the LUP_A4 (top) the matrix contains very few detrital elements and bleaching structures predominate (Figure 2c, d). The matrix of both samples has a composition ranging from boehmitic to feebehmitic to kaolinitic. In the LUP_A4 sample, as in the previous samples, oolites consist predominantly of boehmite (Figure 3d). Boehmitic oolites and hematite fragments are common in the LUP_A15 sample (Figure 3e). In addition, hematite and goethite often occur in crusts and dendriform concretions between the boehmitic oolites. In LUP_A4, Goethit outweighs hematite (Table 1). Compaction structures can be observed in both samples (Figure 3f). Similar to the Villeveyrac sample, the detrital minerals are mainly represented by zircon and rutile. Rare monazites and xenotimes have also been detected.
The two samples from Loupian have high-contrast chemical compositions (Table 1). The sample LUP_A15 has a higher concentration of Al2O3 (66,40% by weight) and a lower amount of Fe2O3 (13,30% by weight) than the sample LUP_A4 (55% by weight and 24,90% by weight, respectively). The amounts of SiO2 and TiO2 are very similar in both samples (about 2,4% by weight and about 3,5% by weight, respectively). Gallium is approximately 70 mg / kg in both samples, V is more abundant in LUP_A4 than in LUP_A15 (469 vs. 377 mg / kg), while Zr has a higher concentration in LUP_A15 (1117 mg / kg). The total amount of REE + Y is higher in the sample LUP_A4 (739 mg / kg) than in the sample LUP_A15 (341,66 mg / kg). For chondrite normalized REE patterns, all samples have approximately the same Eu / Eu * ratio (about 0,65), while the Ce / Ce * ratio provides contrasting values. In particular, the sample LUP_A4 (at the top of the profile) shows a positive Ce anomaly (Ce / Ce * (cho) = 1,94), while LUP_A15 has a slightly negative Ce anomaly (Ce / Ce * (cho) = 0,96). Interestingly, no secondary authentic REE minerals (eg, cerianit and REE fluorocarbonates) were detected in the analyzed samples.
3.2. Mineral Composition and Laser Ablation (LA) -ICP-MS analyzes
The mineral compositions were evaluated by SEM-EDS and LA-ICP-MS analyzes. The analyzes were carried out on Al-hydroxide (boehmite) and Fe-oxyhydroxide (goethite and hematite) masses, which were as homogeneous as possible (under backscattered electrons, BSE) and / or inclusion-free. However, due to the microgranular texture and porosity of the materials studied, in some instances the resulting compositions are far removed from the stoichiometric formulas of boehmite, hematite, and goethite, as accepted by the International Mineralogical Association (IMA). The concentrations resulting from the LA-ICP-MS analysis are shown in Figure 4 and Figure 5 with box plots and detailed in the supplementary material (Tables S2 and S3).
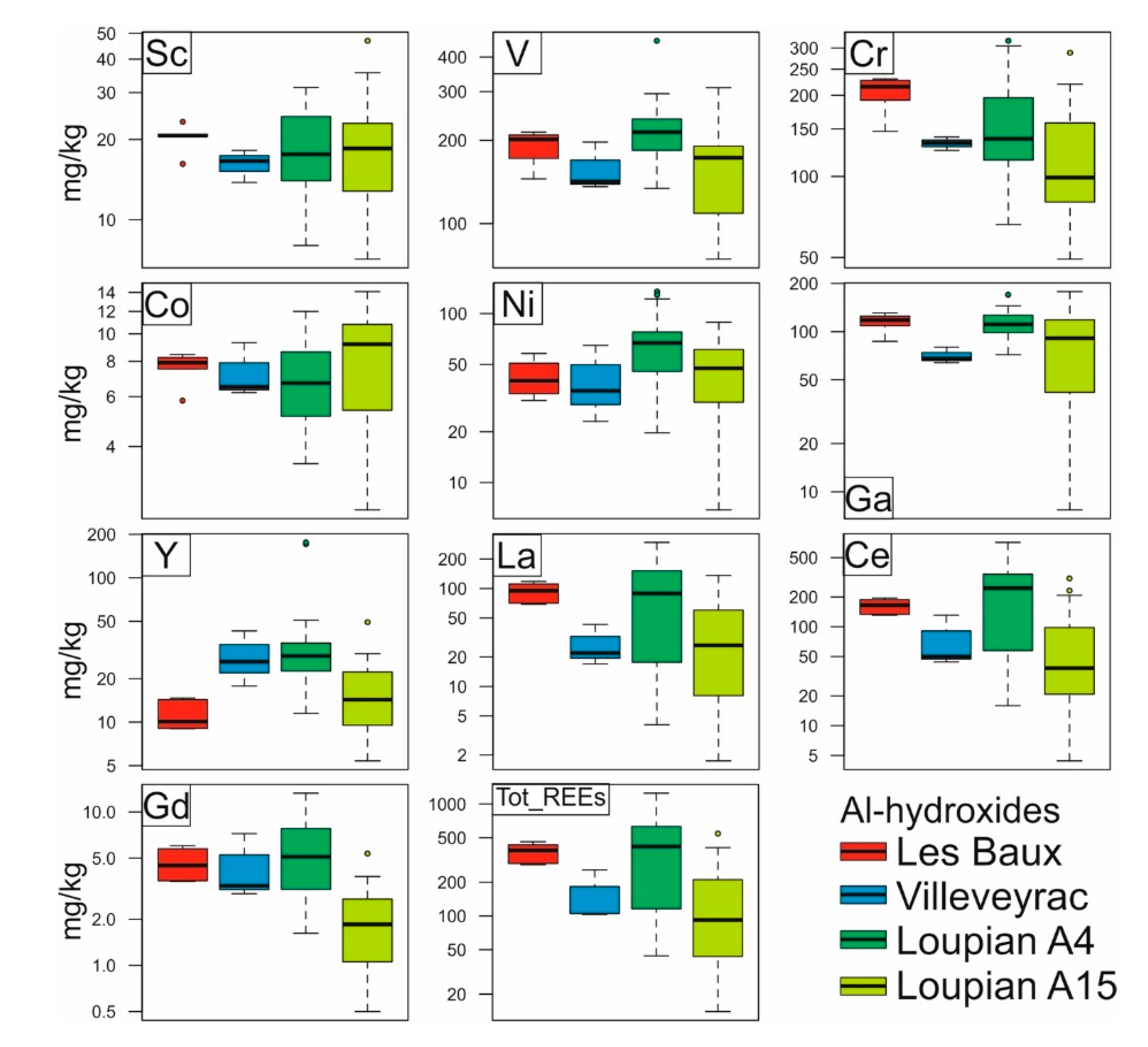
Figure 4. Box plots reporting the compositions (mg / kg) of selected elements in the analyzed Al hydroxides. The individual LA-ICP-MS analyzes are listed in table S2.
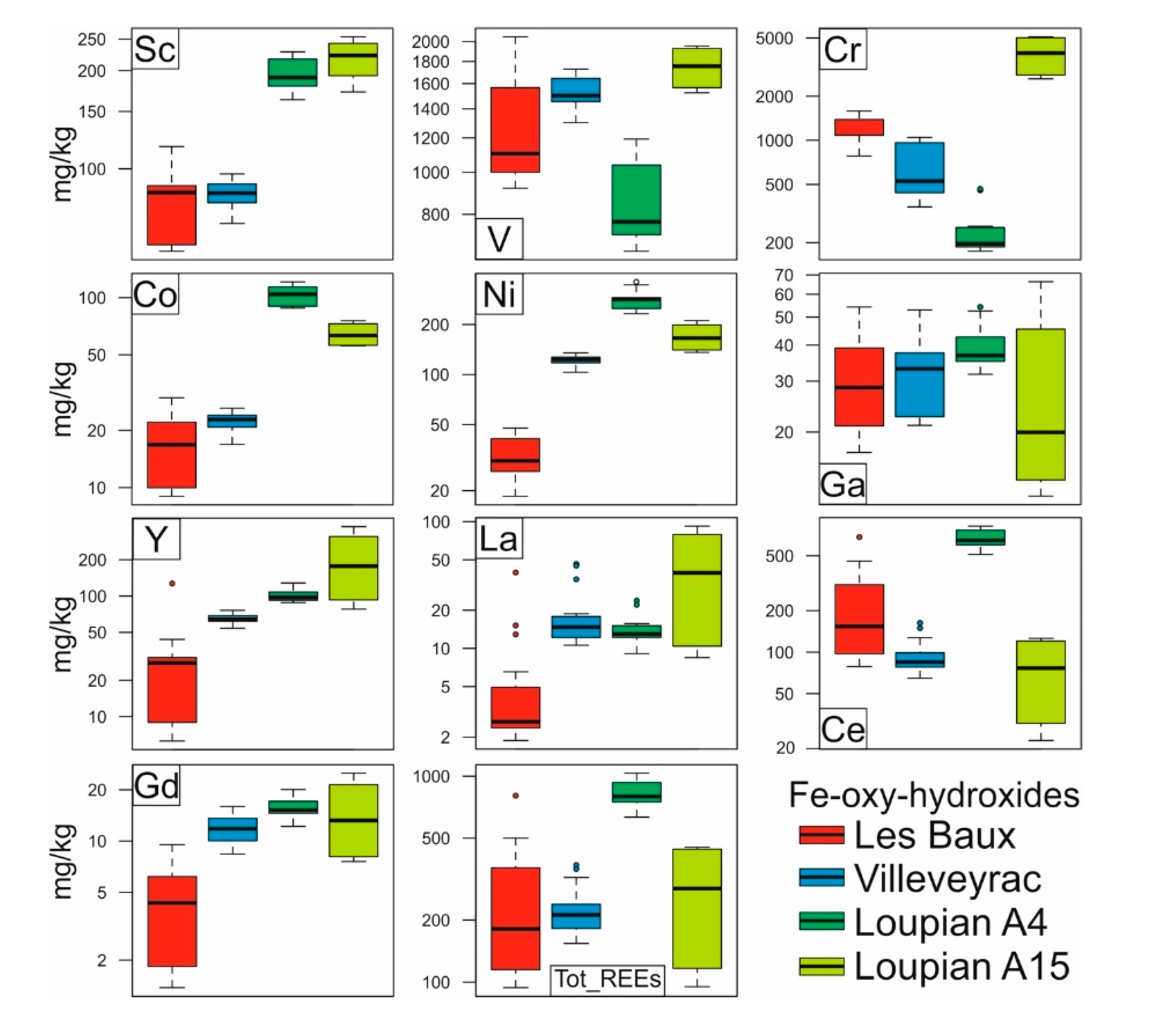
Figure 5. Box plots reporting the compositions (mg / kg) of selected elements in the analyzed Fe-oxy-hydroxides. Individual LA-ICP-MS analyzes are listed in Table S3.
3.2.1. Al hydroxides
In the Les Baux sample, boehmite is finely grown with Fe oxyhydroxides, which leads to very Fe-rich compositions (Fe2O3> 8-10% by weight) under SEM-EDS. In the Villeveyrac sample, boehmite coexists exclusively with kaolinite. This is reflected in many SEM-EDS analyzes in high amounts of SiO2 (5-12% by weight). For this reason, only the SEM-EDS spots with less than 7% by weight Fe2O3 or 5% by weight SiO2 were analyzed with LA-ICP-MS in these samples. In the Loupian samples, the interdependence between boehmite and other minerals is very limited. Inclusionless (under the SEM) boehmite usually shows SiO2 concentrations below 1% by weight, Fe2O3 below 5% by weight (on average about 2,5% by weight) and TiO2 below 1% by weight. Aluminum oxide (Al2O3) shows an average concentration of 78% by weight (stoichiometric boehmite, which is accepted by IMA, has Al2O3 = 83% by weight).
Although the principal elemental compositions of boehmite from the tested samples appear to be differently affected by the factors mentioned above, the minor and trace element concentrations measured by LA-ICP-MS are consistent throughout the samples tested. Among the measured bauxitophilic elements, the highest concentrations are V (average concentrations (average) in the analyzed samples from 180 to 200 mg / kg), Cr (average in the analyzed samples from 100 to 220 mg / kg), Ni (average in the analyzed samples from 45 to 60 mg / kg) and Ga (average in the analyzed samples from 90 to 110 mg / kg) (Figure 4). Because of the high number of points analyzed (more than 40), the Loupian samples have the largest standard deviation among the samples analyzed (Figure 4).
Interestingly, REE levels were also detected in all analyzed Al hydroxides, with mean total REE concentrations of: 374 ± 74 mg / kg at Les Baux, 156 ± 88 mg / kg at Villeveyrac, 395 ± 296 mg / kg at Loupian A4 and 150 ± 145 mg / kg for Loupian A15 (Figure 4). The chondrit-normalized diagrams (Figure 6a-d) showed REEs patterns accompanied by accumulation of LREEs (La to Nd), MREEs (Sm to Tb) and HREEs (Dy to Lu), and common light positive Ce of negative Eu anomalies. In addition, the REE distribution in Al hydroxides in the Les Baux and Villeveyrac samples, as the bauxite normalized graphs show, is on average similar to that of the respective bauxite samples (Figure 7a-d). HREEs are slightly depleted with respect to LREEs in the Les Baux sample, while they have low enrichment in the Villeveyrac sample. For the Loupian Al-hydroxides, the REEs / bauxite patterns are more complex, with relatively flat MREE and HREE patterns and variably enriched LREE segments (Figure 7e-h). In particular, the Al hydroxides are characterized by negative Ce anomalies in the sample at the top of the profile, while the lower profile sample has positive Ce anomalies. The concentrations of La and Ce have a perfect positive correlation, as do Ce and Pr (except for the Fe-rich boehmite from Les Baux, Figure 8).
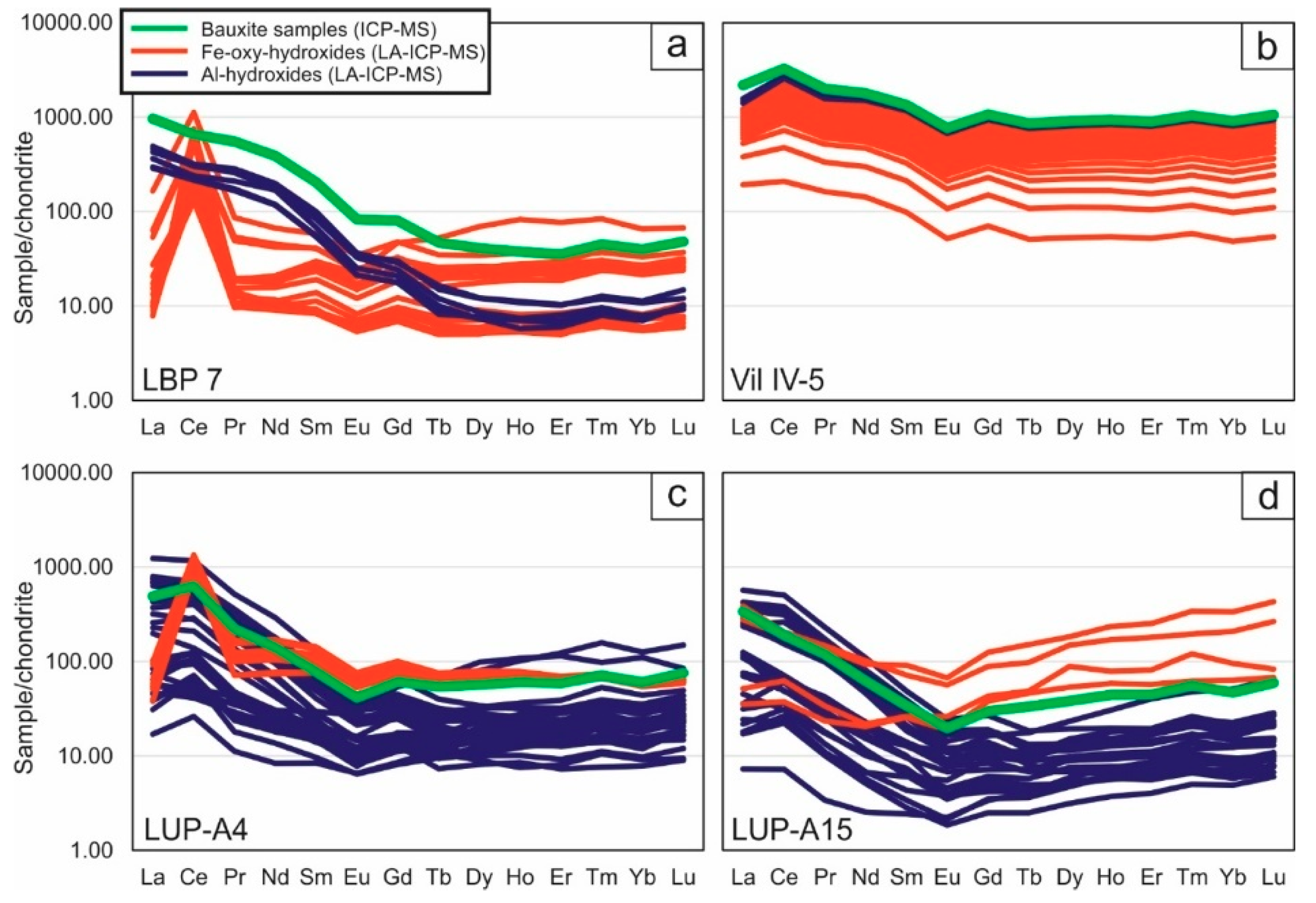
Figure 6. REE concentrations obtained on bulk bauxite samples and in-situ LA-ICP-MS analyzes of Al and Fe (oxy) hydroxides normalized to chondrite [33]: (a) Les Baux -de-Provence; (b) Villeveyrac; Loupian: Samples at the (c) top and at the (d) bottom of the profile.
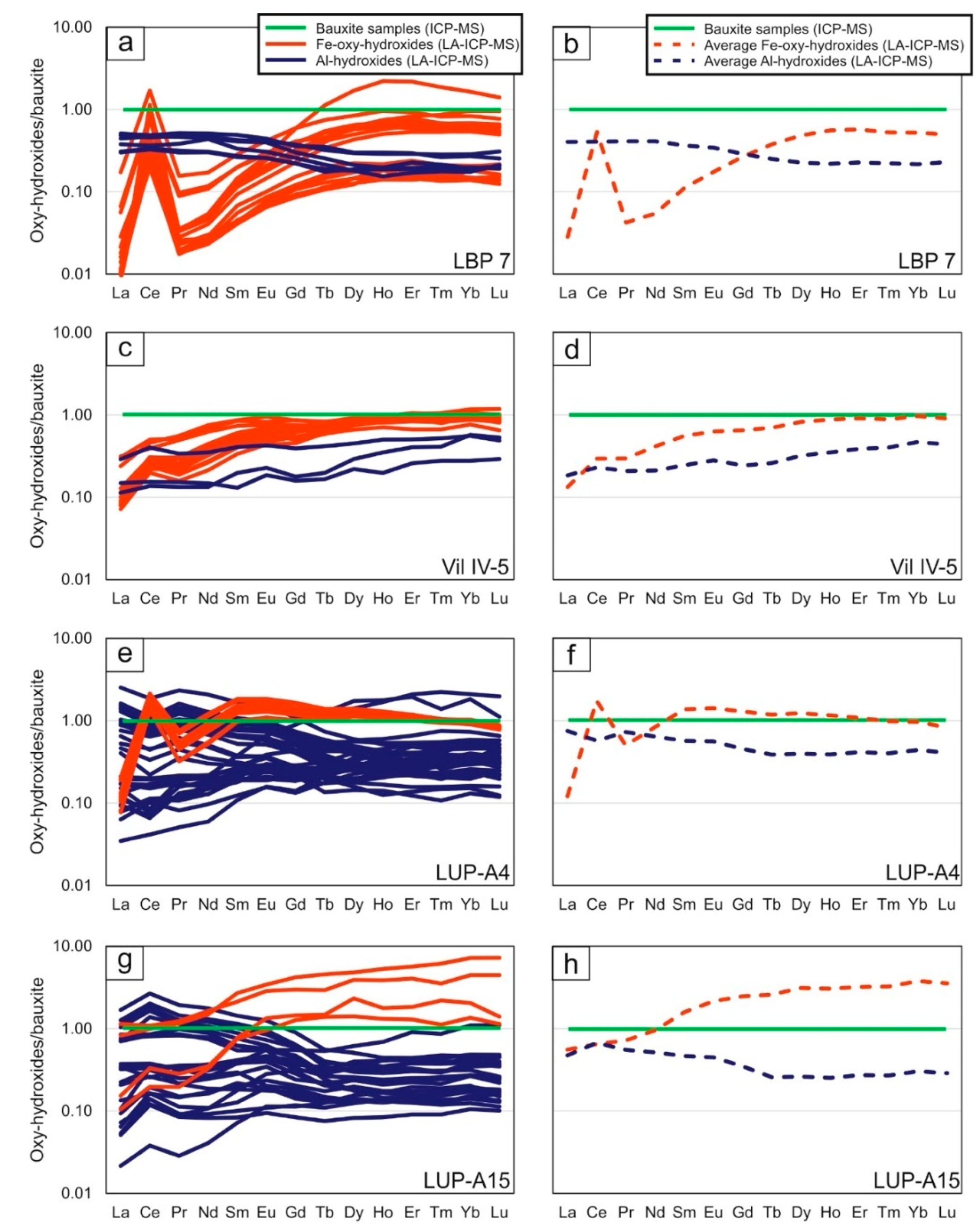
Figure 7. REE concentrations obtained by in situ LA-ICP-MS analysis normalized to the corresponding bauxite bulk samples. Les Baux-de-Provence Al- and Fe- (oxy) -hydroxide (a) single data and (b) average compositions; Villeveyrac Al- and Fe- (oxy) -hydroxide (c) single data and (d) average compositions; Loupian: top of the profile Al- and Fe- (oxy) -hydroxide (e) individual data and (f) average compositions; Loupian: below in profile Al and Fe (oxy) hydroxide (g) individual data and (h) average compositions.
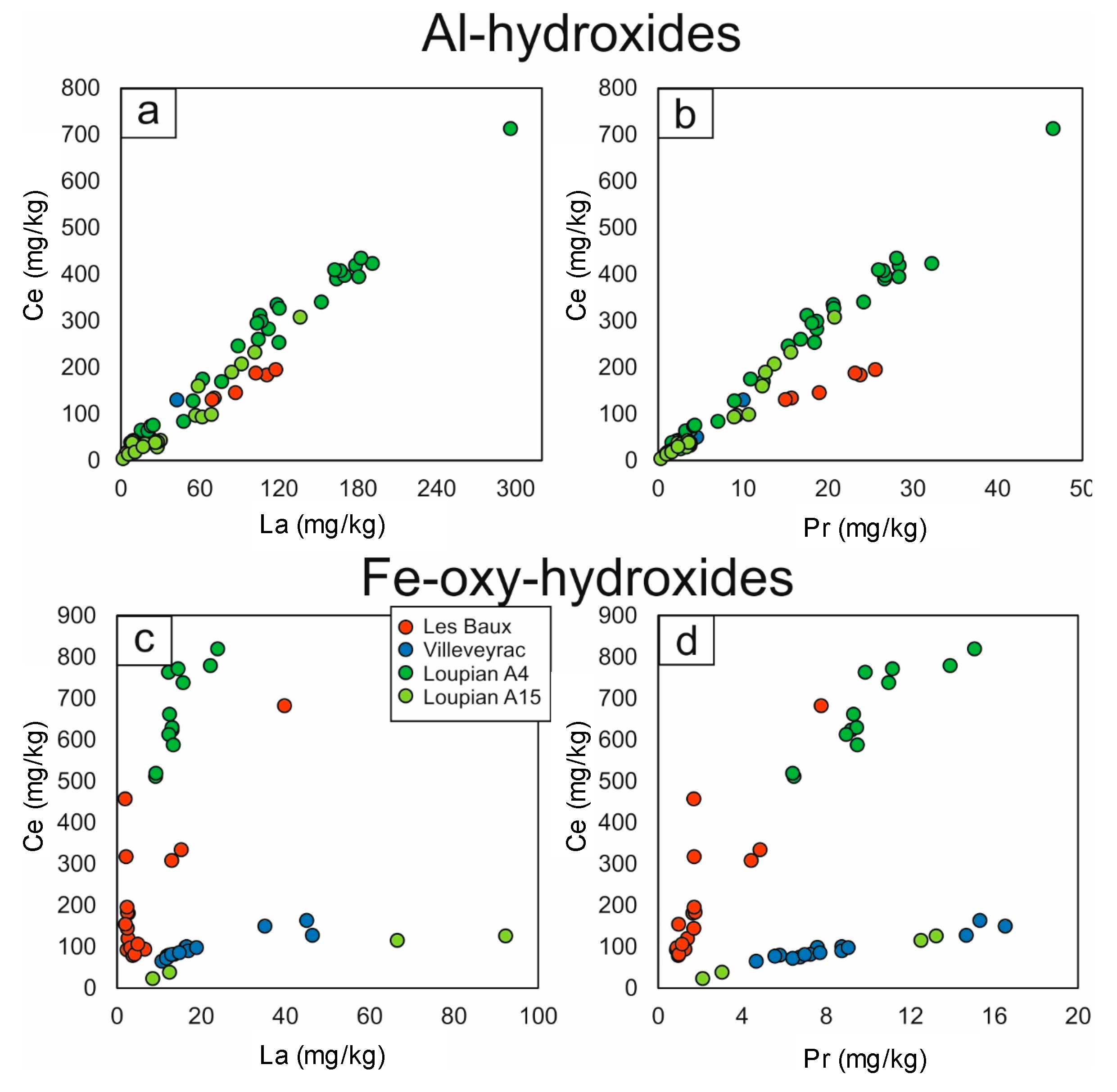
Figure 8. (a) La vs. Ce and (b) Ce vs. Pr (mg / kg) binary plots of in-situ LA-ICP-MS analyzes on Al hydroxides; (c) La vs. Ce and (d) Ce vs. Pr (mg / kg) binary plots of in-situ LA-ICP-MS analyzes on Fe-oxy-hydroxides.
3.2.2. Fe oxy-hydroxides
The major and minor constituents of Fe-oxy-hydroxides were analyzed in all samples tested. In the Les Baux, Villeveyrac and Loupian A15 samples, most hematite analyzes were performed. In all measured SEM-EDS spots, however, Fe (average 90% by weight Fe2O3), Al2O3 (average = 3,4% by weight), SiO2 (average = 0,9% by weight) and TiO2 (average = 1,9 wt .-%) also proved. In the Loupian A4 sample, goethite was the analyzed Fe-oxy-hydroxide: avg. Fe2O3 = 80,8% by weight, avg. Al2O3 = 2,2% by weight, avg. SiO2 = 1,1% by weight and avg. TiO2 = 0,3% by weight. As with the Al hydroxides, the measured chemistry in the Fe mineral phases does not match the compositions accepted by the IMA.
Based on the mineralogy of the investigated Fe minerals, it is possible to detect several different properties of the samples. The hematite of Loupian A15 has the highest concentrations of bauxitophilic elements among the analyzed samples: average 220 mg / kg Sc, average 1750 mg / kg V, average 4400 mg / kg Cr, average 60 mg / kg Co and average 180 mg / kg Ni. The hematite Les Baux is at the other extreme, with the lowest concentrations of the same elements. Interestingly, the goethite of Loupian A4 is characterized by compositions in these elements that are different from all the hematites analyzed. In particular, this goethite has very low levels of V (average 800 mg / kg) and Cr (average 200 mg / kg), and fairly high levels of Ni (average 230 mg / kg) and Co (average 110 mg / kg) (Figure 5) , Gallium has similar concentrations in all measured Fe-Oxy hydroxides (average 35 mg / kg) (Figure 5).
Fe-oxy hydroxides also contain REEs. In particular, the total REE amounts are 254 ± 188 mg / kg for Les Baux, 230 ± 66 mg / kg for Villeveyrac, 827 ± 131 mg / kg for Loupian A4 and 279 ± 188 mg / kg for Loupian A15 (Figure 5). However, in contrast to the Al hydroxides, these concentrations are dominated by Ce. In addition, it is possible by means of La vs.. Ce and Ce vs. Pr binary diagrams (Figure 8) show that the concentrations of these elements in the analyzed Fe-oxyhydroxides follow decoupled positive linear arrangements. In particular, the Loupian samples collected at the base (A15) and at the top (A4) of the bauxite profile show the lowest and highest Ce concentrations, respectively. Considering the REE distribution in the chondrone-normalized spider diagrams (Figure 6), it can be seen that the Loupian A4 Goethite has positive Ce anomalies, while the Loupian A15 hematite has slight negative or no Ce anomalies, coherent with the bulk density. Rock analyzes characterized by similar Ce anomalies in the chondrite-normalized patterns.
The REE distribution in Fe-oxy-hydroxides generally does not match the respective all-rock oleochemical composition in all the samples analyzed (more clearly in the LBP 7 sample, less in the Vil IV-5, Figure 6). This is better illustrated in bauxite normalized patterns (Figure 7), where the Fe-oxyhydroxides have flat MREE and HREE patterns with variable enrichment values across the LREEs relative to the respective bauxites, and strong positive Ce anomalies in the LBP 7 and LUP-A4 samples ,
4. discussion
In the present study, LA-ICP-MS analyzes of Al and Fe (oxy) hydroxides were performed, showing that these minerals contain variable amounts of Al, Fe, Si, Ti as well as trace elements (Ga, Ni, V, Cr, REEs). Although the presence of small amounts of Fe, Si and Ti in Al-hydroxides and Al, Si and Ti in Fe-oxy-hydroxides could be an indication of mineral entanglements, the same elements can also be applied to the same concentrations as adsorbed cations Hydroxide surfaces occur [37,38,39,40]. If one of the selected LA-ICP-MS spots corresponded to a mineral aggregation (eg Fe-oxy-hydroxides, anatase or kaolinite, which are finely fused with boehmite), this should have had only a small influence on the corresponding trace element concentrations, simply because the amount of major hydroxide strongly dominates over a potential mixed mineral. At the same time, trace element concentrations in the Al and Fe (oxy) hydroxides are unlikely to be related to trace element mineral phases (eg, REE minerals), as their presence is likely to be peaks or cracks in LA-ICP-MS time-resolved crude CPS Signal that was not observed.
The data presented show that both Al and Fe (oxy) hydroxides play a significant role in the definition of REE distribution in bauxite, along with the other REE-bearing phases (eg, monazite and xeno-time) normally associated with of this deposit species [1,2,3]. In the investigated samples, Al-hydroxides show on average an accumulation in LREEs, which is similar to the mass rock observed REE distribution. On the contrary, Fe-Oxy hydroxides are enriched in MREEs and HREEs and consumed in LREEs, with the exception of Ce, which always has large positive anomalies. The REE deportation into bauxite deposits has generally been considered to be primarily influenced by the formation of REE phases such as Ce oxides and REE fluorocarbonates [22,26,41]. Previous work [14,15,22,41,42] has shown that the formation of REE-containing minerals causes fractionation between Ce and the other LREEs and HREEs. This process results in a variable REE distribution within the bauxite profiles, with the highest values alternatively being observed either at the top or bottom of the profiles, mainly in response to two different types of species:
- Ce enrichment at the top of bauxite profiles: This property is mainly due to the Ce oxidation from a trivalent to tetravalent state, which is associated with the precipitation of cerianit [(Ce4 +, Th) O2] [42]. Ceroxidation and the resulting CeO2 deposition can occur in the pH range of 5-6 [42].
- LREEs Enrichment at the bottom of the profiles: It is explained by the per descensum model. This can be described as (i) REEs leaching at the top of the profile due to acidic soil solutions (a decrease in the pH of the surface solutions, possibly due to a decrease in ionic strength, can cause the conversion of Ce4 + to Ce3 + in the uppermost part of the karst deposit) (ii) downsizing of the REE-bearing fluids (REE3 + transported in solution as fluoride complexes), (iii) acid buffering through the footwall limestones, and (vi) LREEs and Ce3 + fixations in neoformated fluorocarbonates [26,41,42].
A limited number of studies have suggested that the formation of Fe-oxy-hydroxides in connection with the above processes could also have effects on REE deportment [9,42,43]. For example, Mongelli [42] proposed that rare earths in the Apulian bauxites were trapped with Fe oxides from the Ce-terminated percolation solutions that originate from cerianite precipitation. This process has been proposed to explain abnormal concentrations of REEs in hematitic oolites without proper REE minerals. The REE distribution in hematite oolites was characterized by negative Ce anomalies. Gamaletsos et al. [9] suggested that the occurrence of REE anomalies in Fe-rich bauxites, relative to Fe-poor bauxites in Greek deposits, could be related to either the presence of Ce oxides or epigenetic Ce sorption in Fe oxides. The distribution of REEs in Fe-oxy-hydroxides in bauxites in southern France confirms the suggestions proposed in the literature mentioned: All LA-ICP-MS analyzes determined REE concentrations were above the detection limits and showed that REEs are effective with Fe-Oxy- Hydroxides can be associated, probably as adsorbed cations [44]. In contrast to Mongelli [42], however, the Fe oxyhydroxide crusts and oolites analyzed were always characterized by positive Ce anomalies. They were also enriched with MREEs and HREEs compared to the bulk bauxite, which, according to the SEM-BSE observation, in turn led to freedom from cerianite. Experimental studies [44] have effectively shown that the Fe oxyhydroxides can adsorb high amounts of Ce, MREEs and HREEs under acidic conditions (pH <6) compared to the other LREEs (ie La and Pr). Basic pH values increase the adsorption of the other LREEs and thus mask the Ce anomaly [44]. For the reasons given above, the REEs division observed in this study, and particularly in the sample collected from the top of the Loupian profile (LUP_A4), suggests that REE intercalation in Fe oxy-hydroxides under oxidizing conditions and took place below pH <6.
due to cerium ion precipitation in a pH range between 5 and 6 (eg, [42]), our data strongly suggest that the oxidation of Ce3 + to Ce4 + under the same environmental conditions not only with cerium ion formation but also with Ce4 + incorporation / Adsorption in / on Fe-oxy-hydroxides may be connected. The HREE enrichment and the less obvious positive Ce anomaly in the LUP-A15 Fe oxyhydroxides may be related to the presence of Ce-terminated percolation solutions at the lower end of the bauxite horizon.
In all the samples tested, the Al hydroxides show a general accumulation of LREEs compared to chondrite [33] but also with respect to the corresponding Fe-oxyhydroxides (see the samples LBP 7, LUP_A4 and LUP_A15). To the best of our knowledge and belief, the inclusion of REE in Al-hydroxides was never mentioned in the literature on bauxite deposits. There are, however, several studies [45,46,47,48] dealing with experimental studies on adsorption of metals to Al hydroxides at ambient temperatures, air pressure and variable pH. Fraihurst and Warwick [45] investigated the sorption of Eu, as an analogy to other trivalent cations, to goethite and boehmite, in the presence or absence of humic acids. Eu adsorption on both minerals in the absence of humic acids was shown to exhibit a non-linear increase in pH, with a strong increase in adsorption at pH 4-6 for boehmite and at pH 3-5,5 for goethite. The presence of variable concentrations of humic acids modifies this adsorption scheme, resulting in a general increase in Eu adsorption at low pH (<5 to 6) and a general decrease in adsorption at medium and high pH. This behavior was recently confirmed by Kraemer et al. [47]. Granados-Correa and co-workers [40] confirmed that adsorption is pH-dependent and that the amount of adsorbed metals increases at pH values between 3,5 and 5 when investigating Cd (II) adsorption on boehmite and goethite , They also found that boehmite is characterized by a higher adsorption efficiency than goethite. This was considered to be related to the surface of boehmite, which is larger than that of goethite, and a larger number of functional groups contains [40]. Therefore, the present literature supports the possibility that trivalent cations, such as the REEs, can be adsorbed on Al-hydroxides, much like the better-understood Fe-oxy-hydroxides. Considering the REE concentrations in the French bauxites in general, and in particular the LBP 7 sample, it appears that Al-hydroxides preferentially incorporated LREEs (except Ce) in relation to the HREEs as opposed to Fe-oxyhydroxides. This is not surprising since in the pH range in which cation adsorption on boehmite is preferred (pH 4 to 6 [45]), the Fe-oxyhydroxides preferably contain Ce and HREEs [44]. Further features can be observed in the Loupian profile, where Al-hydroxides in the bauxite-normalized diagrams show almost mirrored REE patterns compared to Fe-Oxy-hydroxides. Especially at the top of the Loupian profile, where Fe-Oxy hydroxides are characterized by positive Ce anomalies and high HREE concentrations, the Al hydroxides have Ce-attenuated and LREE-enriched patterns. At the bottom of the profile, the Al hydroxides are still enriched on average with LREEs, but also show small positive Ce anomalies, while Fe-Oxy hydroxides show strong HREE enhancements without Ce anomalies.
Somewhat different is the case of the sample Vil IV-5, in which both Fe-oxy-hydroxides and Al-hydroxides have similar chondrite-normalized patterns (Figure 6b) and differ only in that the former are richer in MREEs and HREEs than the latter (Figure 7c, d). Therefore, unlike the other samples, Al-hydroxides in Vil IV-5 appear to lack LREE accumulations. This particular feature could result from a combination of different processes: (i) the formation of authentic LREE minerals in uninvestigated sections of the deposit that produced LREE-used solutions from the outset, which later became apparent in the anomalous patterns of Al-hydroxides [42], or (ii) the in situ acidic leaching of bauxite (also aided by the appearance of kaolinite), which has gradually consumed the more reactive Al hydroxides relative to the Fe-Oxy-hydroxides [40] , However, a more thorough and extensive investigation is needed to determine which processes affected the Villeveyrac deposit.
From an application perspective, the most relevant finding of the present study is the detection of REEs adsorbed to Al hydroxides. As mentioned in the introduction, it is currently being discussed whether bauxite deposits and / or red muds are to be considered as potential REE resources [4,22,23,24,25,26,27,28]. In a recent paper on Greek red mud [27], it has been shown that during the Bayer process, while Ga is mainly accumulated in process liquors, rare earths tend to remain unaffected in solid phases and to fully penetrate the bauxite residue all in the form of a new LREE ferrotitanate phase [(REE, Ca, Na) (Ti, Fe) O3], derived from the digestion of already existing rare earth minerals originally contained in bauxite feed [27]. However, it could also be shown that possible existing REE-containing minerals (eg monazite, REE fluorocarbonates) also occur as untouched mineral phases in the bauxite test [27]. Although we have not analyzed French red mud, this suggests, analogously to the data presented in this paper, that the mentioned LREE ferrotitanate was not produced by digestion / reaction of former monazites or REE fluorocarbonates, but by REEs, Fe, and Ti, which were originally contained in Al-hydroxides and were released into the liqueur after their digestion. If confirmed, this would also indicate that REEs adsorbed to Al hydroxides rapidly form compounds with other free elements present in the liquid upon release. Therefore, an interesting topic to be developed in the future is whether (and how) REEs released during Al hydroxide digestion can be efficiently recovered from the pregnant liquor, as is common with Ga.
5. Conclusions
- LA-ICP-MS analyzes of Al-hydroxides and Fe-oxy-hydroxides performed on four samples of French bauxites revealed that these minerals contain variable amounts of Al, Fe, Si, Ti, and trace elements (Ga, Ni, V , Cr, REEs).
- Al-hydroxides show an accumulation in LREEs that resembles the mass rock observed REE distribution. Fe-oxy hydroxides are instead enriched with MREEs and HREEs and consumed in LREEs, with the exception of Ce, which always has large positive anomalies. REEs are likely associated with the Al and Fe (oxy) hydroxides as adsorbed cations.
- The data presented strongly suggest that positive Ce anomalies observed in chondrite normalized samples of bauxite bulk samples not only reflect the formation of cerium ions but also the oxidation of Ce3 + to Ce4 + and Ce4 + incorporation / adsorption in / are connected to Fe-oxy-hydroxides.
- Although the present study has been conducted on a limited number of samples, the occurrence of rare earths in Al hydroxides is of considerable interest because it is likely that rare earths will be released into the pregnant fluid along with Al during the digestion step of the Bayer process become. Therefore, the use of a particular collector has potential for efficient recovery of REE as routinely performed on Ga.
Complementary materials
The following information is available online at https://www.mdpi.com/2075-163X/9/9/504/s1.
Author Contributions
Conceptualization, NM, FP and LS; Software, NM, FP, MT; Validation, NM, FP, MT; Methodology and formal analysis, GB, AM and LS; Investigation, NM, FP, CC-M., SC…., GS and MT; Data curation, NM, FP; Creation of original drafts, NM, FP; Review and editing of texts, CC-M., MB; Project management, NM; Financing Acquisition, NM, LS
Financing
The research that led to these results was funded by the “Program per il finanziamento della ricerca di Ateneo 2016-Progetto CEB” awarded to Nicola Mondillo by the Università degli Studi di Napoli Federico II (Italy). Additional funds from the European Union's Horizon 2020 research and innovation program come from a Marie Skłodowska-Curie Individual Fellowship (project name GOSSAN, number 751103), which is awarded to R. Herrington and supports the L. Santoro scholarship.
Acknowledgments
The authors are grateful to R. de'Gennaro and J. Buret for help and SEM and LA-ICP-MS analyzes, respectively. Our appreciation also goes to the three anonymous reviewers whose comments have significantly improved the quality of the paper. G. Mongelli is thanked for the editorial work.
conflicts of interest
The authors do not declare a conflict of interest.
References
Bárdossy, G .; Aleva, GJJJ Laterite Bauxite; Elsevier: Amsterdam, Netherlands, 1990; 624p. (Google Scholar)
Bárdossy, G. Karst Bauxite, Bauxite deposits on carbonate rocks (developments in economic geology); Elsevier: Amsterdam, Netherlands, 1982; P. 441. (Google Scholar)
Bonuses, M .; Rollinson, G .; Mondillo, N .; Balassone, G .; Santoro, L. Quantitative mineralogical characterization of karstic exhaust deposits in the southern Apennines, Italy. Econ. Geol. 2013, 108, 813-833. [Google Scholar] [CrossRef]
Lee Bray, E. Bauxite and alumina. In mineral commodity summaries; US Geological Survey: Reston, VA, USA, 2019. (Google Scholar)
Hind, AR; Bhargava, SK; Grocott, SC Surface chemistry from Bayer Process solids: A review. Colloidal surfing. A physicochem. Closely. Asp. 1999, 146, 359-374. [Google Scholar] [CrossRef]
Mongelli, G .; Bonuses, M .; Oggiano, G .; Mameli, P .; Sinisi, R .; Buccione, R .; Mondillo, N. Critical Metals Distribution in Tethyan Karstbauxit: The Cretaceous Italian ores. Ore Geol. Rev. 2017, 86, 526-536. [Google Scholar] [CrossRef]
Abedini, A .; Calagari, AA REE geochemical properties of titan-rich bauxites: The Perm Kanigorgeh horizon, NW Iran. Turk. J. Earth Sci. 2014, 23, 513-532. [Google Scholar] [CrossRef]
Gamaletsos, PN; Godelitsas, A .; Mertzimekis, TJ; Divine, J .; Steininger, R .; Xanthos, S .; Berndt, J .; Clamp, S .; Kuzmin, A .; Bárdossy, G. Thorium partitioning in Greek industrial bauxite, examined by synchrotron radiation and laser ablation techniques. Nucl. Instrum. Methods Phys. Res. Res. Sect. B beam interaction. Hook. At the. 2011, 269, 3067-3073. [Google Scholar] [CrossRef]
Gamaletsos, PN; Godelitsas, A .; Filippidis, A .; Pontikes, Y. The potential of the rare earth elements of Greek bauxite active mines in terms of sustainable rare earth demand. J. Preserve. Metal. 2018, 5, 20-47. [Google Scholar] [CrossRef]
Hanilçi, N. Geological and geochemical development of bauxite deposits Bolkardaği, Karaman, Turkey: transformation of slate into bauxite. J. Geochem. Explore. 2013, 133, 118-137. [Google Scholar] [CrossRef]
Karadag, MM; Küpeli, Ş .; Arik, F .; Ayhan, A .; Zedef, V .; Döyen, A. Rare Earth Element (REE) Geochemistry and genetic implications of the Mortaş (Seydişehir / Konya-Southern Turkey) bauxite deposit. Chemistry. Earth 2009, 69, 143-159. [Google Scholar] [CrossRef]
Ling, KY; Zhu, X.-Q .; Tang, HS; Wang, ZG; Yan, HW; Han, T .; Chen, WY Mineral properties of karstic bauxite deposits in Xiuwen Ore Belt, Central Guizhou Province, Southwest China. Ore Geol. Rev. 2015, 65, 84-96. [Google Scholar] [CrossRef]
Mameli, P .; Mongelli, G .; Oggiano, G .; Dinelli, E. Geological, geochemical and mineralogical features of some Nurxite bauxite deposits (Western Sardinia, Italy): Insights into the conditions of formation and parental relationship. Int. J. Earth Sci. 2007, 96, 887-902. [Google Scholar] [CrossRef]
Mondillo, N .; Bonuses, M .; Balassone, G .; Rollinson, G. Karst Bauxite in the Campanian Apennines (Southern Italy): A New Approach. Point. Mineral. 2011, 80, 407-432. (Google Scholar)
Putzolu, F .; Piccolo Papa, A .; Mondillo, N .; Bonuses, M .; Balassone, G .; Mormon, A. Geochemical characterization of bauxite deposits from the mining region of Abruzzo (Italy). Minerals 2018, 8, 298. [Google Scholar] [CrossRef]
Torró, L .; Proenza, YES; Aiglsperger, T .; Bover-Arnal, T .; Villanova-de-Benavent, C .; Rodríguez-García, D .; Ramírez, A .; Rodríguez, J .; Mosquea, LA; Salas, R. Geological, geochemical and mineralogical characteristics of REE bearing Las Mercedes bauxite deposit, Dominican Republic. Ore Geol. Rev. 2017, 89, 114-131. [Google Scholar] [CrossRef]
Wang, Q .; Deng, J .; Liu, X .; Zhang, Q .; Sun, S .; Jiang, C .; Zhou, F. Discovery of REE minerals and their geological significance in the Quyang bauxite deposit, West Guangxi, China. J. Asian Earth Sci. 2010, 39, 701-712. [Google Scholar] [CrossRef]
Yu, W .; Wang, R .; Zhang, Q .; You, Y .; Chen, Y .; Liang, Y. Mineralogical and geochemical development of the Fusui bauxite deposit in Guangxi, southern China: From the original Permian orebody to a quaternary, saline-like deposit. J. Geochem. Expl. 2014, 146, 75-88. [Google Scholar] [CrossRef]
Zarasvandi, A .; Charchi, A .; Carranza, EJM; Alizadeh, B. Karst bauxite deposits in the Zagros mountain belt, Iran. Ore Geol. Rev. 2008, 34, 521-532. [Google Scholar] [CrossRef]
Jaskula, BW Gallium. In mineral commodity summaries; US Geological Survey: Reston, VA, USA, 2019. (Google Scholar)
Authier-Martin, M .; Forte, G .; Ostap, S…;