Tungsten [ˈvɔlfram] is a chemical element with the element symbol W and the ordinal number 74. It is one of the transition metals, in the periodic table it is in the 6th subgroup (group 6) or chromium group. Tungsten is a shiny white heavy metal of high density, which is brittle in its pure state. Of all pure metals, it has the highest melting point and the second highest boiling point. Its best-known use is therefore the filament in light bulbs.
As early as the 16th century, the Freiberg mineralogist Georgius Agricola described the occurrence of a mineral in Saxon tin ores, which made tin extraction considerably more difficult due to the slagging of the tin content. The part of the name “wolf” comes from this property, since the mineral “ate” the tin ore like a wolf. Whether it was wolframite is still controversial today, as he spoke of the “lightness” of the mineral. He named the mineral lupi spumumwhich translated from Latin means "wolf (s) foam". It was later called Wolfram, from mhd. R.A.M "Soot, dirt", as the black-gray mineral can be easily ground and then reminds you of soot. Its chemical symbol W comes from tungsten.
The common word in English, Italian and French Tungsten derives from Tung Sten (Swedish for "heavy stone"). This did not mean that Wolfram itself (Swedish Volfram), but called calcium tungstate. In 1781 the German-Swedish chemist Carl Wilhelm Scheele recognized a previously unknown salt in this. Pure tungsten was first produced in 1783 by the Spanish brothers Faust and Juan José Elhuyar (who worked under Scheele's direction) by reducing tungsten trioxide, which is extracted from wolframite.
occurrence
The tungsten content of the earth's crust is around 0,0001 g / t or 0,0064 percent by weight (Clarke value). So far, the metal could not be proven in nature (in pure form). The "Doklady Akademii Nauk" in Russia published a report on solid tungsten in 1995 without this being examined by the IMA's "Commission on New Minerals, Nomenclature and Classification" (CNMNC). Some minerals, especially oxides and tungstates, are known. The most important tungsten ores are wolframite (Mn, Fe) WO4 and Scheelite CaWO4. There are also other tungsten minerals, such as Stolzit PbWO4 and Tuneptit WO3 · H2O.
The largest deposits can be found in China, Peru, the USA, Korea, Bolivia, Kazakhstan, Russia, Austria and Portugal. Tungsten ores can also be found in the Ore Mountains. The safe and probable world deposits are currently 2,9 million tons of pure tungsten.
The most important known occurrence of tungsten in Europe is in the Felbertal in the Hohe Tauern (state of Salzburg in Austria).
Promotion worldwide
In 2006 the world production of pure tungsten was 73.300 tons. By far the largest producer of tungsten is China. More than 80% of the tungsten produced in the world is made there. The states with the highest production of tungsten (2006):
| Rank | Country | Delivery rates (in tons per year) |
|---|---|---|
| 1 | China | 62.000 |
| 2 | Russian Föd. | 4.500 |
| 3 | Canada | 2.500 |
| 4 | Austria | 1.350 |
| 5 | Portugal | 900 |
| 6 | North Korea | 600 |
| 7 | Bolivia | 530 |
| 8 | other countries | 900 |
Promotion in Austria
In Austria, the tungsten ore scheelite was first discovered in 1815/16 on the Schellgaden gold deposit in the municipality of Muhr (state of Salzburg). As a result, beautiful scheelite crystals, sometimes several centimeters in size, were found in many crevices of the Hohe Tauern. All of these finds were of no practical use. The large deposit in the Felbertal remained undiscovered for the time being.
In 1950 it became known that large amounts of scheelite appeared in the magnesite deposit on the Wanglalm near Lanersbach / Tux (Tyrol) in the rear of the Zillertal, which had been being mined since 1927. It was coarse scheelite intergrown with magnesite and quartz. In the following years around 10.000 tons of ore with an average tungsten oxide content of 1,8% were extracted, which represented a high quality that is unique in the world. Because of the low market price, tungsten extraction was stopped at the end of the 1960s, but resumed in 1971 and continued until the magnesite mine was closed in 1976.
1967 was eventually discovered the largest Scheelitvorkommen Europe in the Felbertal. The ore pieces present in the streams were traced using UV light (Scheelite fluorescents). The difficult exploration work in the high alpine terrain (highest mining site on Brentling in 2100 at sea level) began 1971, the initially overground mining was recorded in Felbertal 1976 (from 1979 also underground mining, surface mining 1986 set). From the beginning of 1993 to the middle of 1995, mining was temporarily suspended due to the low market price of tungsten.
Tungsten ore from the Felbertal is processed in nearby Mittersill. From here the scheelite concentrate reaches Sankt Martin im Sulmtal (Styria). On the site of the Pölfing-Bergla underground lignite mine, which was closed in 1976, a tungsten smelter was built, in which tungsten oxide, tungsten metal and tungsten carbide powder has been produced from concentrates from several countries since 1977.
The most important German processors are HC Starck and the Longyear GmbH.
Extraction and presentation
Tungsten cannot be obtained from oxidic ores by reduction with coal, as this results in tungsten carbide.
Adding an ammoniacal solution creates a complex called ammonium paratungstate (APW). This is filtered off and then converted into relatively pure tungsten trioxide at 600 ° C. Tungsten (VI) oxide (WO3), which is reduced to steel-gray tungsten at 800 ° C under a hydrogen atmosphere:
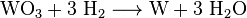
This creates gray tungsten powder, which is usually compacted in molds and electrically sintered into bars. At temperatures above 3400 ° C, a compact tungsten metal can be melted in special electric furnaces with a reducing hydrogen atmosphere (zone melting process).
Features
Physical Properties
Tungsten is a shiny white metal that can be stretched in its pure state and is of high hardness, density and strength. The density is almost the same as that of gold, the Brinell hardness is 250 HB, the tensile strength 550-620 N / mm2 (soft) up to 1920 N / mm2 (hard). The metal exists in a stable body-centered cubic α-modification with a lattice plane spacing (= lattice constant) of 316 pm at room temperature. This type of crystal structure is often called the tungsten type. With an as metastable β-modification of tungsten (distorted cubic-body-centered), on the other hand, it is the tungsten-rich oxide W3O.
After the element carbon, tungsten has the second highest melting point of all chemical elements at 3422 ° C. The boiling point of 5555 ° C is only exceeded by the rare metal rhenium with 5596 ° C by 41 K.
The metal is a superconductor with a transition temperature of 15 mK.
Chemical properties
Tungsten is a chemically very resistant metal that is hardly attacked even by hydrofluoric acid and aqua regia (at least at room temperature). However, it dissolves in mixtures of hydrofluoric and nitric acid and molten mixtures of alkali nitrates and carbonates.
isotope
33 isotopes and 5 nuclear isomers are known of tungsten. Of these, 5 isotopes occur in nature 180W, 182W, 183W, 184W and 186W. The tungsten isotope 184W has the greatest frequency. All 5 natural isotopes were considered stable for a long time. It was not until 2004 that the CRESST experiment at the Laboratori nazionali del Gran Sasso succeeded in proving that the isotope was a secondary result of the search for dark matter 180W is subject to alpha decay. The half-life is an extremely long 1,8 trillion years, so this decay cannot be detected in a normal laboratory environment. The radioactivity of this natural isotope is so low that it can be ignored for all practical purposes. In contrast, the artificial radioactive isotopes of tungsten have short half-lives between 0,9 ms 185W and 121,2 days at 181W.
Usage
The most important application of tungsten, because of its high melting point, is in the lighting industry as a filament in incandescent lamps and as an electrode in gas discharge lamps and electron tubes.
In light bulbs, use is made of the fact that the electrical conductivity of tungsten is significantly lower than that of the conductive metals copper and aluminum. As a result, the thin tungsten filament heats up until it glows, while the thicker leads made of the conductive metals hardly get warm.
It has its second great importance as an alloy metal in ferrous metallurgy. It forms tungsten carbides in tool steels, which increase the secondary hardness.
Due to its high density, it is used for balance weights and for shielding against radiation. Although its density and thus the shielding effect is much higher than that of lead, it is used less often than lead for this purpose because it is more expensive and difficult to process. Also, because of the high density of tungsten, some armies use armor-piercing ammunition with a projectile core made of tungsten carbide instead of the cheaper but radioactive and toxic depleted uranium. During the Second World War, tungsten was important for the construction of the German Panzerranate 40, which had a tungsten core. In the future, ammunition with a tungsten core will be used by the new Puma infantry fighting vehicle, which is to replace the Marder.
Because of its high corrosion resistance, tungsten can also be used as a material for equipment in chemical plants. However, because of the poor machinability of tungsten (tungsten can only be welded by laser or electron beam), this embodiment is rarely used. The same applies to a conceivable application in the field of medical technology.
In physiology, especially neurophysiology, tungsten microelectrodes are used for extracellular recordings.
In addition, electrodes for welding processes are manufactured from tungsten. For example, in resistance welding, especially when welding materials such as copper, bronze or brass. Also in universal TIG (Tungsten Inert Gas) welding, an electrode is made of tungsten or an alloy thereof. These electrodes are not melted down during the welding process. The arc burns as plasma in a protective gas between the electrode and the component. The filler material is supplied separately in the form of rods.
In sport, tungsten is used to manufacture high-quality barrels for darts, in archery it is used to make tips for special arrows, and hammer heads are sometimes made of tungsten to reduce air resistance and the rotation radius for hammer throwing. In addition, tungsten plates are used as additional weights in Formula 1 to achieve the prescribed minimum weight of Formula 1 cars (including oil, brake and coolant, as well as drivers in racing overalls and with helmet) of 620 kg (as of 2010). It has also been used by large racers in keel bombs in sailing for some time. The water resistance is greatly reduced due to the greater density compared to conventional materials such as lead or cast iron. There are also tennis rackets that have tungsten fibers incorporated into their carbon fiber frame. In this way, specific areas of the racket frame can be additionally stabilized in order to increase the playing precision.
In fly fishing, nymphs and streamers (bait fished under water) are weighted down with tungsten beads that are pierced and pushed onto the shank of the hook, so that they dive faster and deeper.
Strings for musical instruments are sometimes wound with tungsten in order to increase their weight and thereby reduce the pitch.
Tungsten is also used in X-ray diagnostics as a target material in the anode. The  - and
- and  -Lines of the characteristic X-ray radiation are around 59 keV and 67 keV.
-Lines of the characteristic X-ray radiation are around 59 keV and 67 keV.
In scanning tunneling microscopy, tungsten is often used as the material for the probe tip.
Since the beginning of the 21st century, tungsten carbide, incorrectly referred to as tungsten, has also been used for jewelry (tungsten jewelry), e.g. B. rings processed. This is very easy to determine from the hardness and density. WC has a Mohs hardness of 9,5, tungsten only 7,5. So far, all jewelry parts on the market have been made from tungsten carbide.
physiology
Tungsten is considered a positive bio-element by anaerobic bacteria of the type Eubacterium acidaminophilum used and incorporated as a cofactor in some enzymes. E. acidaminophilum is an amino acid fermenting bacterium which uses tungsten in the enzymes formate dehydrogenase and aldehyde dehydrogenase. In these organisms, tungsten replaces molybdenum because it is much more common in their natural environment (volcanic vents on the sea floor).
toxicology
According to the current state of knowledge, tungsten and its compounds are considered physiologically harmless. Lung cancer among workers in hard metal producing or processing plants is attributed to the cobalt that is also present.
In the animal model it was found that the largest amount of orally ingested tungsten compounds is quickly excreted in the urine. A small part of the tungsten goes into the blood plasma and from there into the erythrocytes. It is then deposited in the kidneys and in the bone system. Three months after administration, most of the very small amounts of tungsten absorbed by the body are found in the bones.
In 2003 in Fallon / Nevada with 16 children suffering from leukemia since 1997 and in Sierra Vista / Arizona with nine children also suffering from blood cancer, two so-called cancer clusters - this is a local area with an above-average rate of cancer cases - were identified. In both places the drinking water has exceptionally high concentrations of tungsten. Significantly increased tungsten concentrations were detected in the urine of the general public. Both places are known for their tungsten ore deposits. In the subsequent investigations by the Center for Disease Control (CDC), which lasted about a year, no direct connection between tungsten and leukemia could be established. Tungsten did not show any carcinogenic effects in any test method, and no cancer clusters were found in other places in Nevada with similarly high tungsten values in the urine of the population.
safety instructions
As a powder or dust, it is easily flammable, non-flammable in a compact form.
Connections
Oxide
Tungsten forms several oxides. Between the initial member:
- Tungsten (VI) oxide WO3 - lemon yellow
and the final member:
- Tungsten (IV) oxide WO2 - brown
Are there any other intermediate oxides?
- W10O29 blue-violet, homogeneity range WO2,92-WHERE2,88
- W4O11 red-violet, homogeneity range WO2,76-WHERE2,73
- W18O49, WHERE2,72
- W20O50, WHERE2,50
Other compounds
- Sodium Tungstate Na2WO4
- Zirconium tungstate ZrW2O8 shows an anomaly when heated.
- Tungsten bronzes MxWO3; M = alkali metal, alkaline earth metal, lanthanoid, approx. 0.3 <x <0.9 have electrical conductivity and are intense and differently colored depending on the metal content.
- Calcium tungstate CaWO4 is known as a mineral under the name Scheelite.
- Tungsten Carbide WC is an extremely hard metal-like compound. There is also Diwungsten Carbide W2C.
- Tungsten hexafluoride WF6
- Lead tungstate PbWO4
- Tungsten disulfide WS2 Use as dry lubricant (Similar to MoS2)
Use of the compounds
Tungsten carbide is used as a neutron reflector in nuclear weapons to reduce the critical mass. Tungsten carbides (hard metal) are used in material processing due to their high hardness.
Tungstates are used to impregnate fabrics to make them flame-retardant.
Tungsten colors are used in painting as well as in the ceramics and porcelain industry.
Lead tungstate is used as a modern scintillator in particle physics.
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, symbol, atomic number | Tungsten, W, 74 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series | Transition metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group, period, block | 6, 6, d | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | greyish white, shiny | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-33-7 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth shell | 64 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| atomic mass | 183,84 u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (193) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 162 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electron configuration | [Xe] 4f145d46s2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. ionization | 770 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. ionization | 1700 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | fixed | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| crystal structure | cubic body-centered | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 19,3 g / cm3 (20 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 7,5 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( = 7,8 10−5) = 7,8 10−5) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| melting point | 3695 K (3422 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 5828 K (5555 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 9,47 · 10−6 m3/ mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 824 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| heat of fusion | 35,4 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| speed of sound | 5174 m / s | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Specific heat capacity | 138 J / (kg · K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 18,52 · 106 A / (V · m) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| thermal conductivity | 170 W / (m K) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| oxidation states | 6, 5, 4, 3, 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| normal potential | −0,119 V (WO2 + 4H+ + 4e- → W + 2H2O) |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| electronegativity | 2,36 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| isotope | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
Hazard H and P phrases H: 228EUH: no EUH ratesP: 210-240-241-280-370+378 Gefahrstoffkennzeichnung
 |
| Light- flammable |
| (F) |
Powder R- and S-phrases R: 11S: 43

