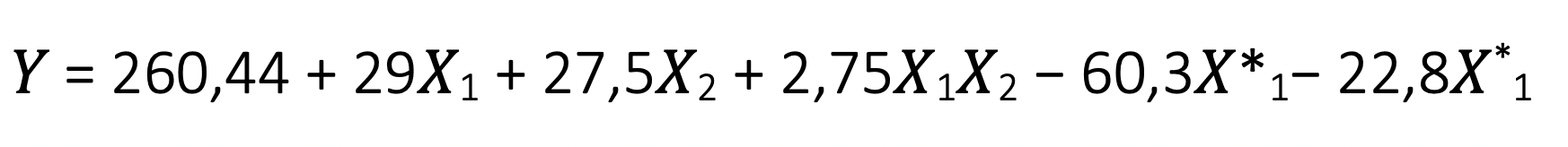Due to numerous inquiries about how ultradispersive copper powder is manufactured, what it is used for and why it is so expensive, we have a scientific paper on this topic translated into German. The translation was done by an employee who is not a professional translator. The content of the German PDF is identical to the Russian original. We could only access a Russian script, as we know that this product is only manufactured in Russia and Chile for commercial purposes. Other countries also produce ultra-dispersive copper powder, but mostly only in their own research laboratories and always only in the required amount.
PRODUCTION OF ULTRADISPERS COPPER POWDERS, STABILIZED WITH WATER-SOLUBLE POLYMERS FOR ANTIFRICTION METAL POLYMER MATERIALS.
Introduction and general characteristics of the work
Composites form a separate section of material.
They form their own production and market segments and sometimes form entire industries. From their areas of application
Ultradisperse and nanoscale powders can be distinguished by their use as fillers in composite materials [1-3].
- The materials obtained in this way are used to manufacture solidified bulk goods (hard alloys, ceramic-metal materials, metal-polymer composite materials).
- Ultradisperse powders improve material performance, in mechanical engineering as lubricant additives, abrasives, membranes, catalysts, adsorbents etc.
- Metal ultradisperse powders are used to manufacture rocket fuels, explosives, pressed and sintered products.
- Powders are used as fillers for the production of effective tread, anti-slip and wear protection materials as well as resource-saving, hydrophobic, self-cleaning and bio-inert composite materials.
- These materials expand the functional and resource possibilities of machines, constructions, products that are used in various branches of industry: in mechanical engineering and construction, in transport, in the energy industry and in the chemical industry.
- Nuclear industry, military equipment, medicine and everyday life.
- Ultradisperse powders, which are used for composite materials with a polymer matrix, enable the production of novel composite nanomaterials with a wide range of practical applications.
- The properties of the powder particles and the materials derived from their use depend not only on the chemical composition, but also on the shape and size of the particles. In the nanoscale range, the ratio of surface to volume particles in contrast to macro and micro particles is measured.
In the nanoscale range, the ratio of surface to volume particles in contrast to macro and micro particles is measured. The properties of ultra-disperse powders can be very different and vary widely when the ratio changes.
Practice explains the wish
The researcher and manufacturer for the production of powders of various types of chemical composition with minimal particle size. Colloid chemistry, which is the study of small particles of substances in liquids and gases, appeared a century and a half ago when an understanding of the importance of powdered substances began to emerge.
The classic materials science scheme "Composition - Structure - Properties" of the academic IV Tananaev.
By introducing the particle size as one of the most important parameters of materials, it was transformed into a scheme of “composition - structure - dispersion - properties”.
A large number of powdery materials arise not only from their type and chemical composition, morphology and particle size, but also from the way they are extracted. All methods of obtaining powders are implemented in two ways: "from top to bottom" and "from bottom to top". The first is the processing of macro-objects, mostly through physical methods that result in the dispersion of the materials. The second method is based on the “construction” of powder particles from atomic and molecular objects, in which case chemical techniques are usually used.
Many technological methods for producing ultradisperse powders are currently known, but there are no universal approaches which allow powders of any type and chemical composition to be produced. Each technology is limited to a particular type of powder by application, and therefore, based on practical need, it is necessary to develop different technological methods for producing different powder materials [8-10].
Relevance of the research topic.
Increasing the operational properties of polymers for plain bearings in friction units of machines and mechanisms by introducing alloy additives into the material means an increase in mechanical and thermal loads. In order to ensure an optimal combination of the physical and mechanical properties of the materials, many fillers are used, but the high filling of such polymers as fluoroplastic - 4 (F-4) and polyethylene - 277 (PE-277) leads to a decrease in the sliding properties of the Material, while improving the resistance to high loads.
The reduction in the sliding properties of the material is mostly due to an uneven distribution.
Filler particles in the entire polymer matrix, which leads to a supersaturation zone and a lack of filler. There are several ways to achieve an even distribution of the filler over the entire volume of the material, one of which is the use of surfactants to stabilize the particles, but this method leads to saturation of the material with by-products, which also negatively affects the Properties of composite materials.
The lack of methods that allow the formation of a chemically inert shell in the production of ultradisperse powders (UDP), which protects the powders from sticking together during the storage and manufacture of compositions, determines the relevance and novelty of the topic of the dissertation indicates the need for special studies.
The work was carried out in the "Engineering Technology" and "Materials Science and Technology of Materials" departments of the South Russian State Polytechnic University named after MI Platov as part of the following activities. Theoretical and technological basics for the development of energy-efficient ways of manufacturing powdery and composite functional materials ”, project number 7.3767.2011.
The goals and tasks of the research are to improve the properties of products made of composite materials made of friction metallic polymer materials by introducing ultra-disperse copper powders that are stabilized with water-soluble polymers.
To achieve this goal, the following tasks had to be accomplished:
- the development of a technology for producing ultra-dispersed copper powder that is stabilized with water-soluble polymers;
- determine the influence of water-soluble polymers, polyacrylamide and polyvinylpyrrhoidone on the properties and characteristics of the obtained ultradispersed copper powder;
- the dependence of the granulometric composition and morphology of ultra-dispersed copper powder on the surface modification must be determined;
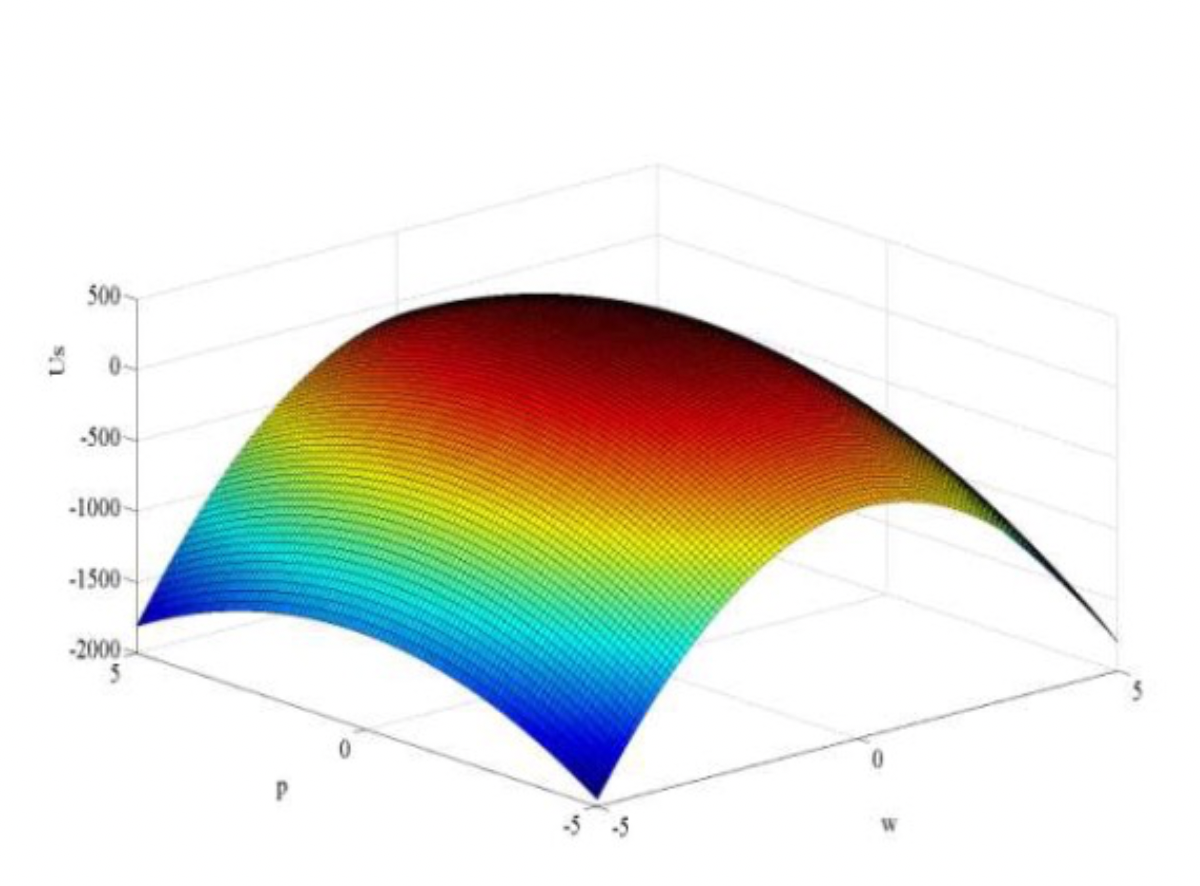
- develop a mathematical model of the force dependency from the technological parameters and use it to determine the optimal amount of nanofillers and the conditions for processing the mixtures;
- the effect of stabilized ultra-dispersed copper powder on the properties of a metallic-polymer composite material due to the evenly distributed ultra-dispersed copper powder in the matrix of the composite materials.
A scientific novelty:
- The proposed method for obtaining ultra-dispersed copper powder by electrolysis in the presence of water-soluble polymers as particle stabilizers differs from the known methods in that it allows the dispersibility of the obtained ultra-dispersed copper powder to be 2-3 times compared to the industrially used methods for obtaining electrolytic Reduce powders and reduce the average size of the obtained ultra-dispersed copper powder, as well as increase the amount of the nanoscale fraction.
- The effect of average particle size reduction in the presence of polyvinylpyrrolidone was noted. This reduces the average size of the powder particles to <50 nanometers and the uniform, mutual distribution of the polymer on a copper surface is achieved.
- It shows the uniform distribution of the particles of the stabilized ultradisperse copper powder in a matrix made of composite material.
In contrast to the methods examined so far, an increase in the adhesive strength of the powder was found.
The particle-matrix relationship that affects the improvement of the physical, mechanical and anti-friction properties of the material.
Practical importance.
A high-performance technology for the extraction of polydisperse ultra-disperse copper powders has been developed.
Scientifically based practical recommendations are given for the selection of the optimal types of intake of nanopowders by the electrolysis method, the compositions of the metal polymer composites and the technology of their intake are developed.
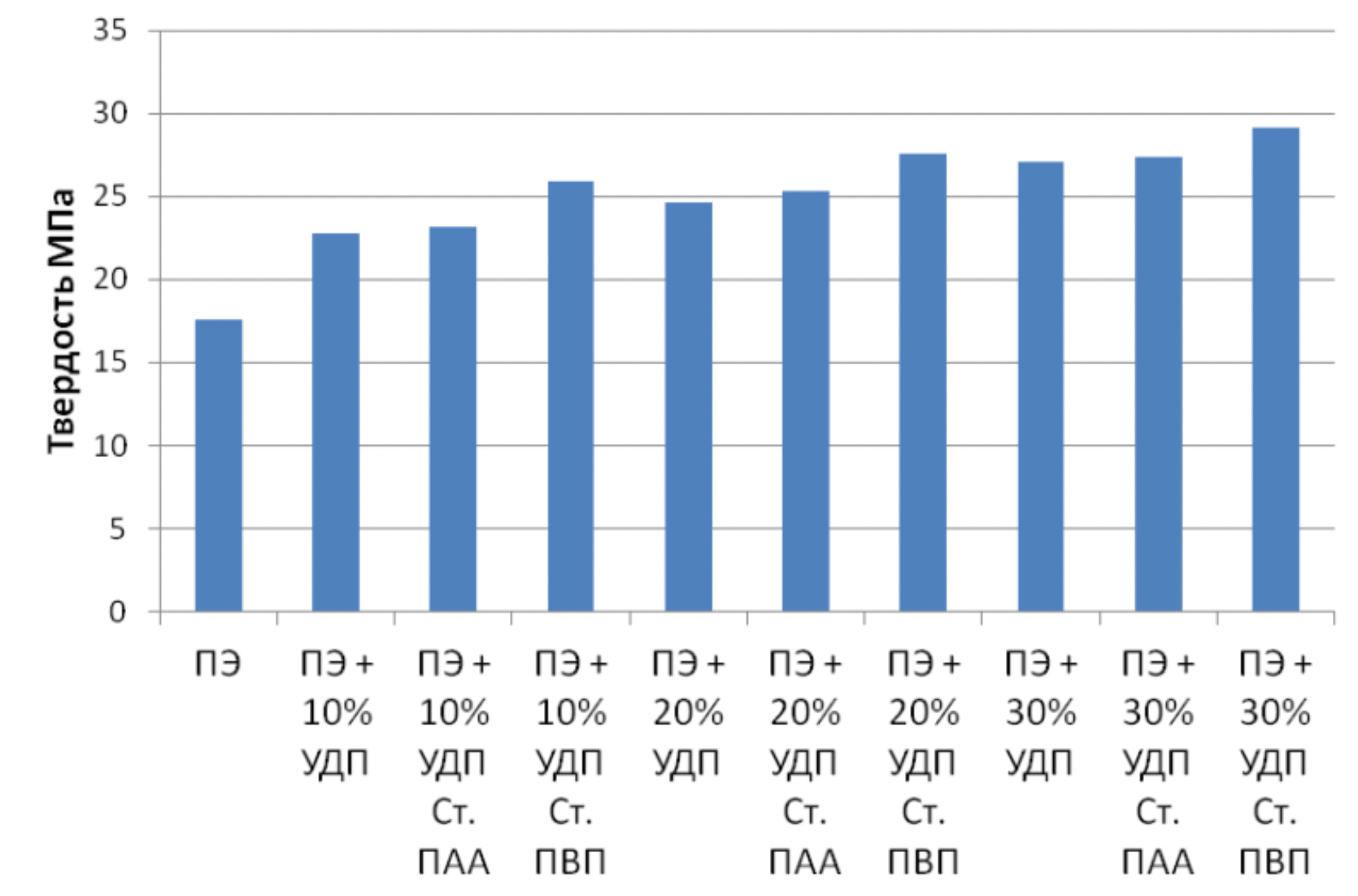
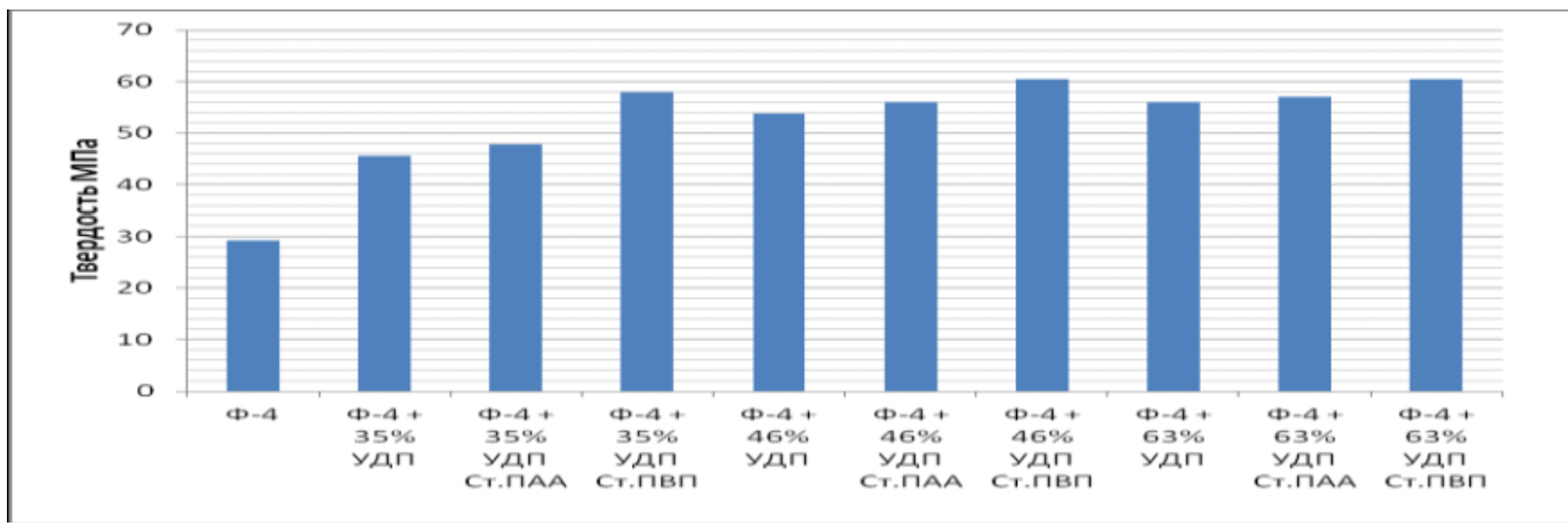
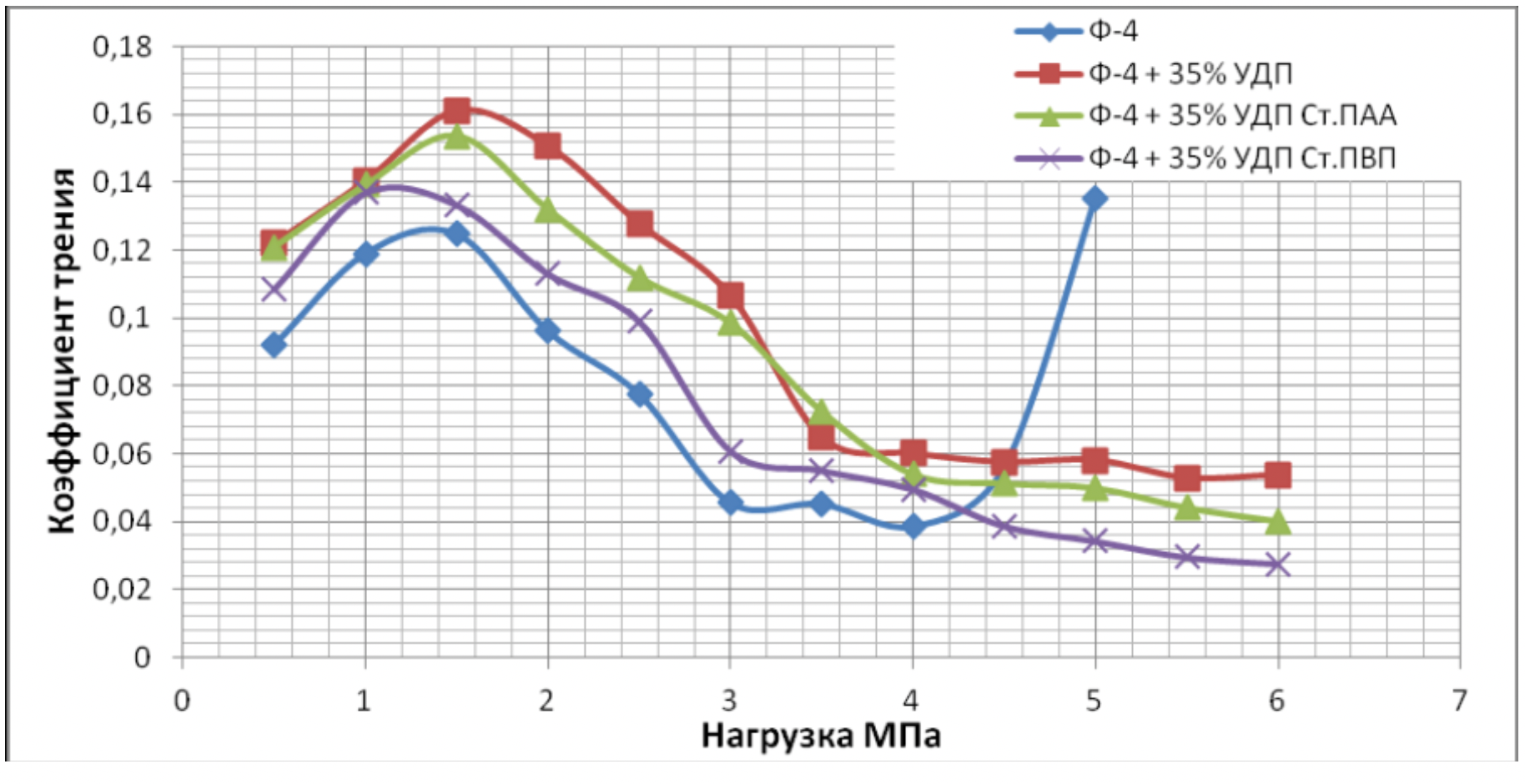
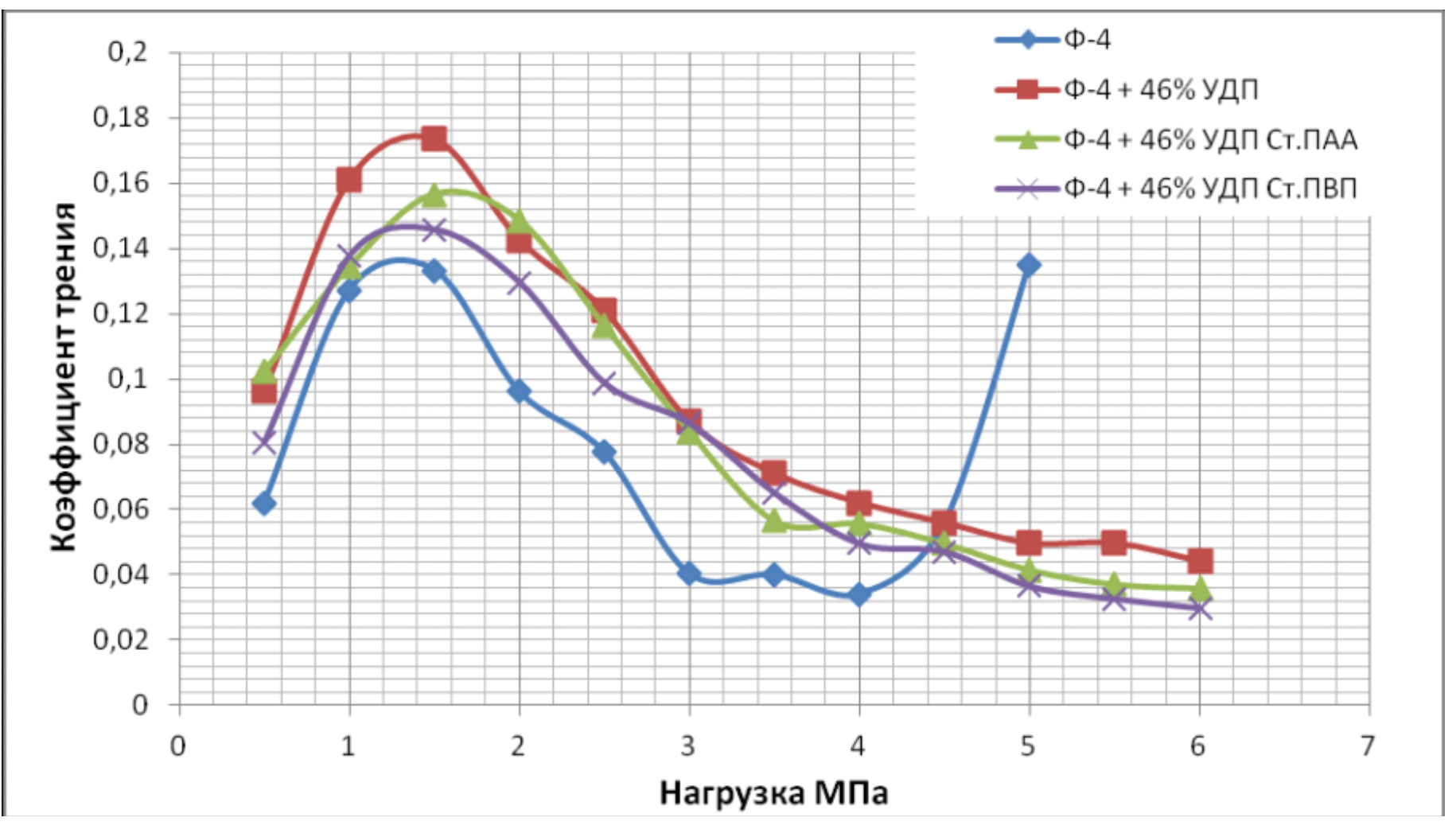
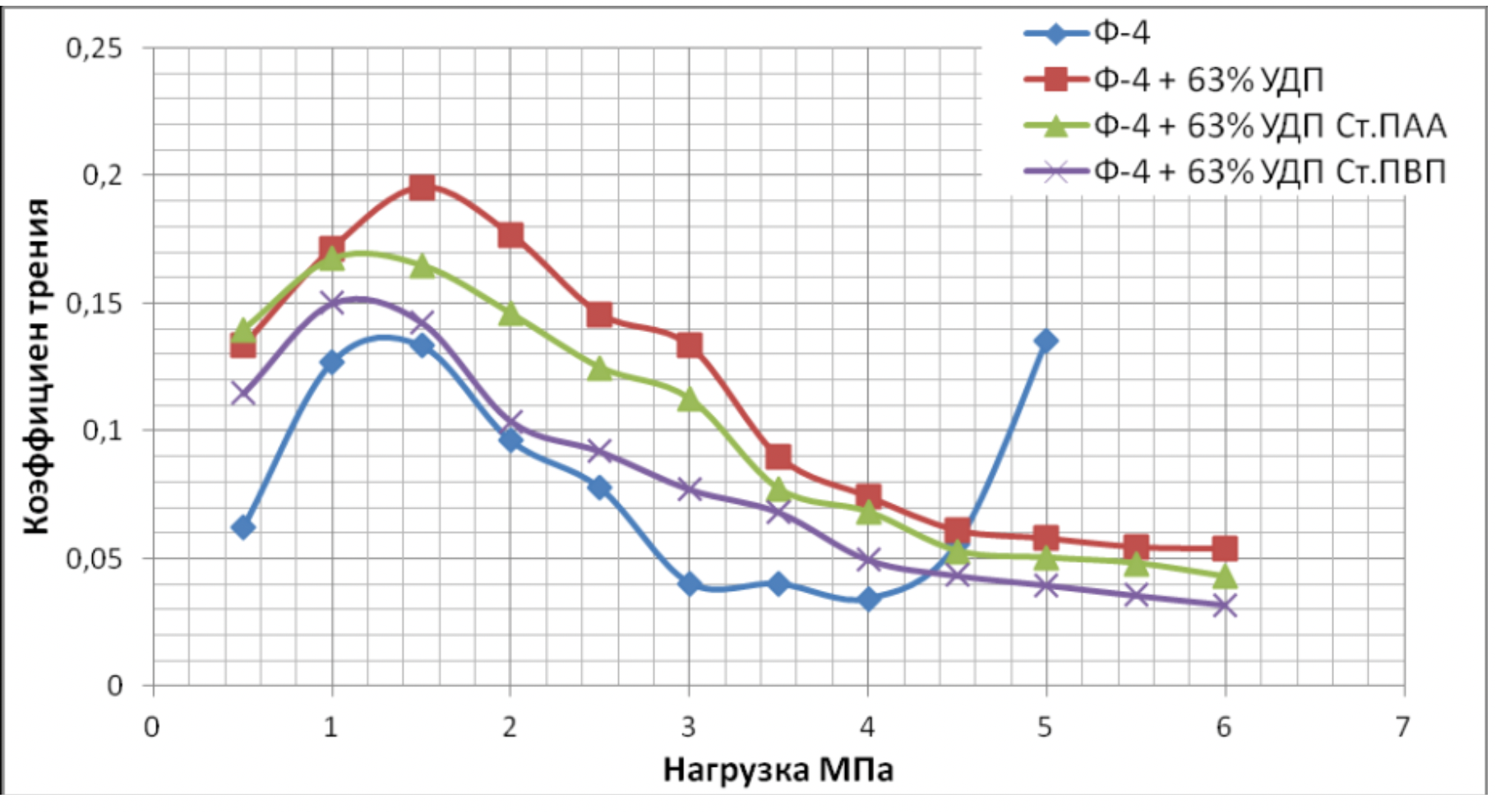
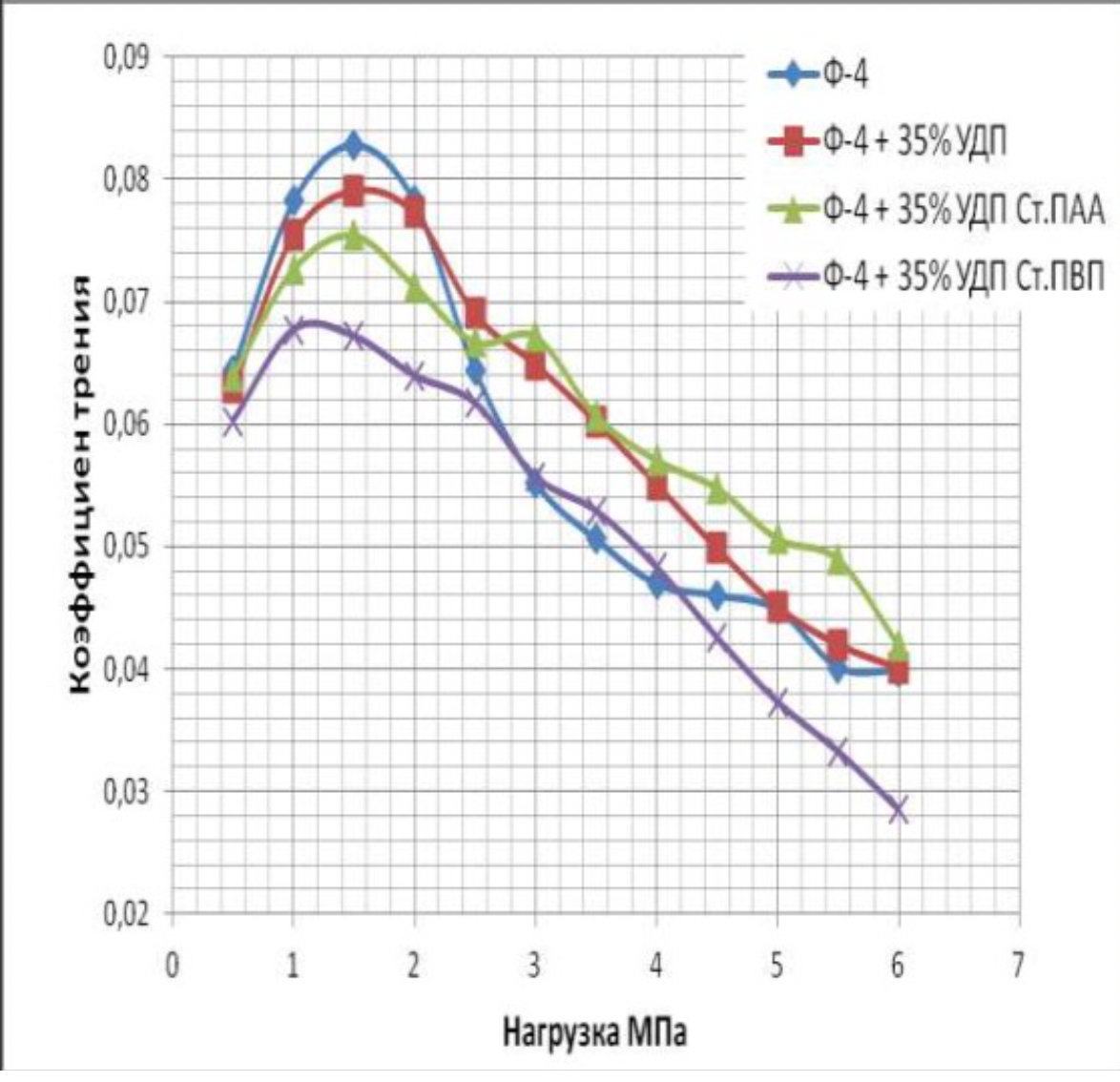
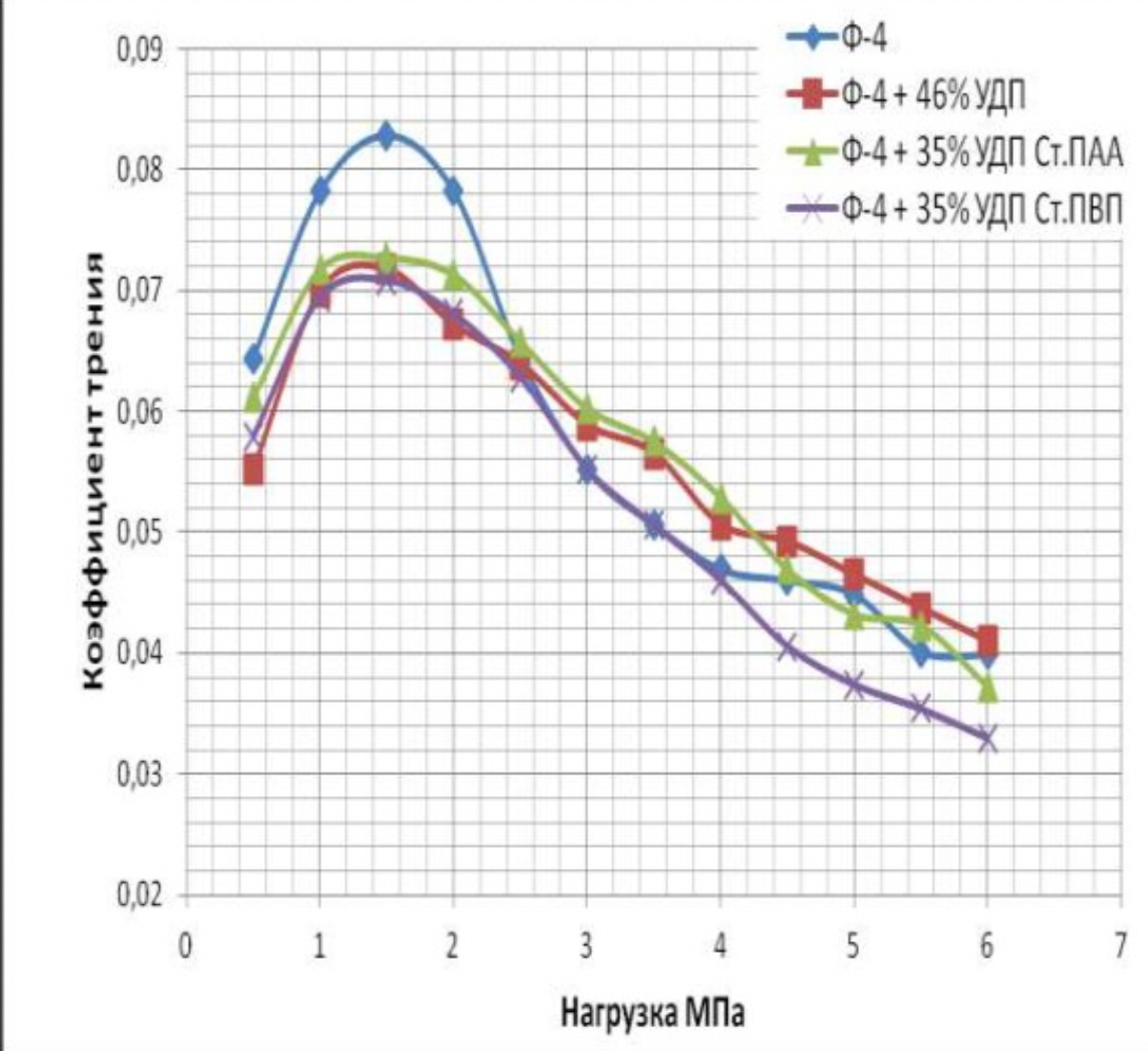
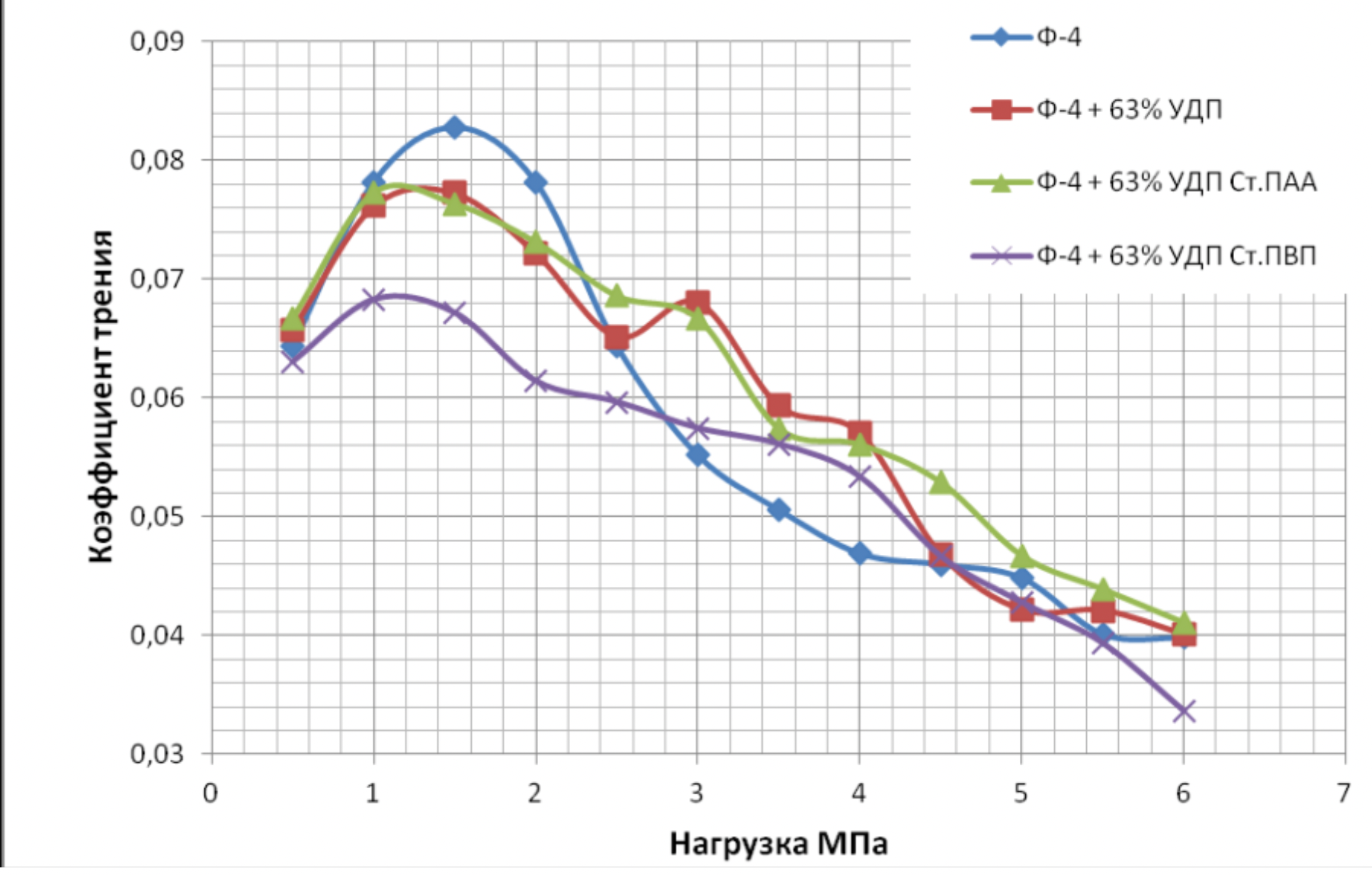
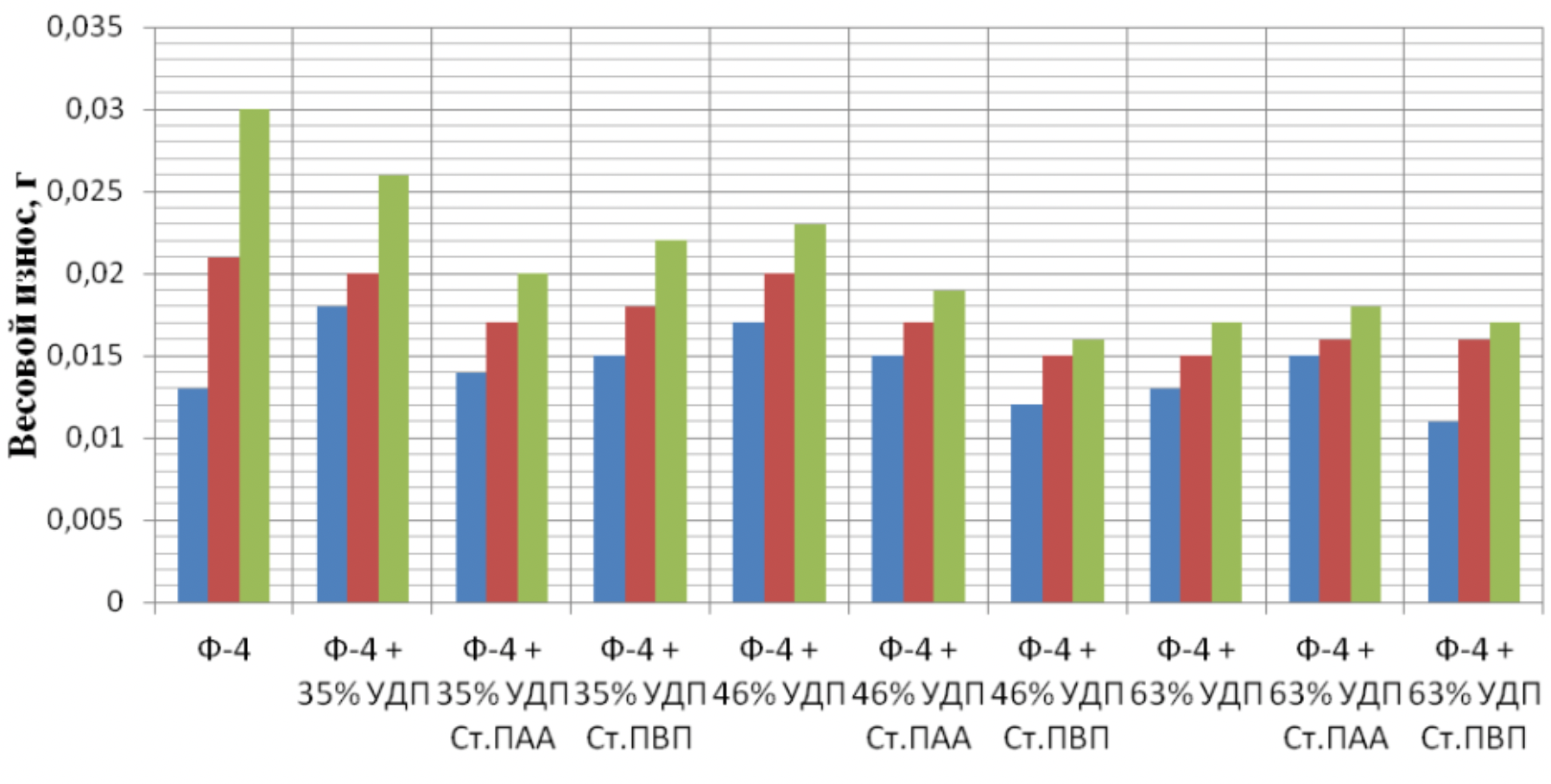
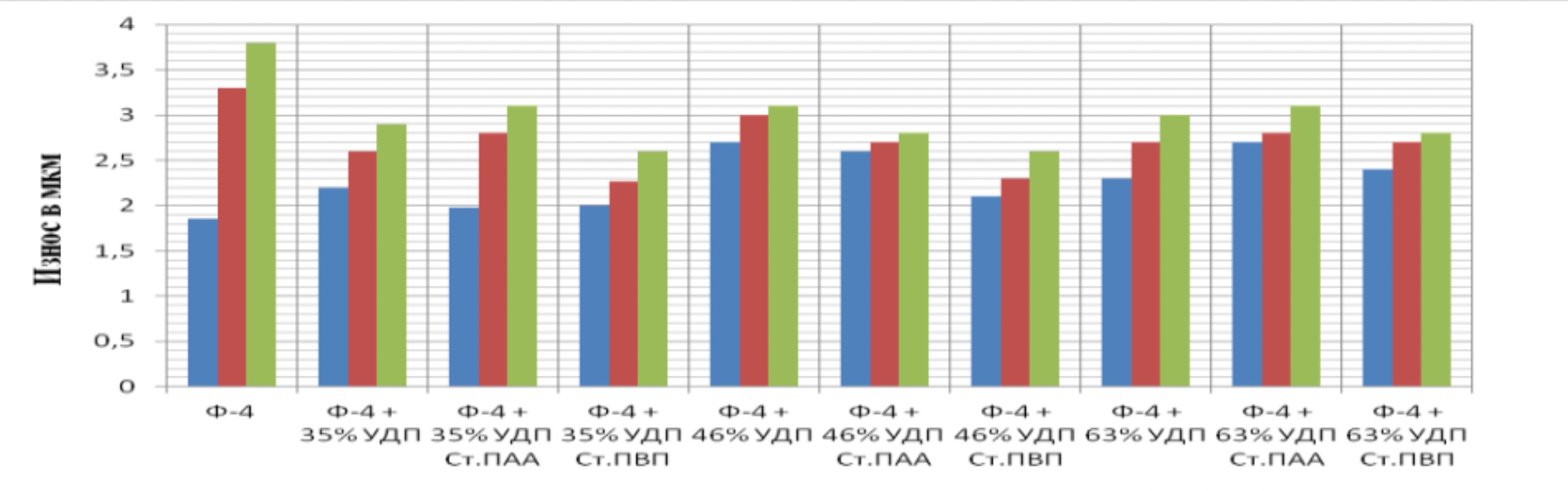
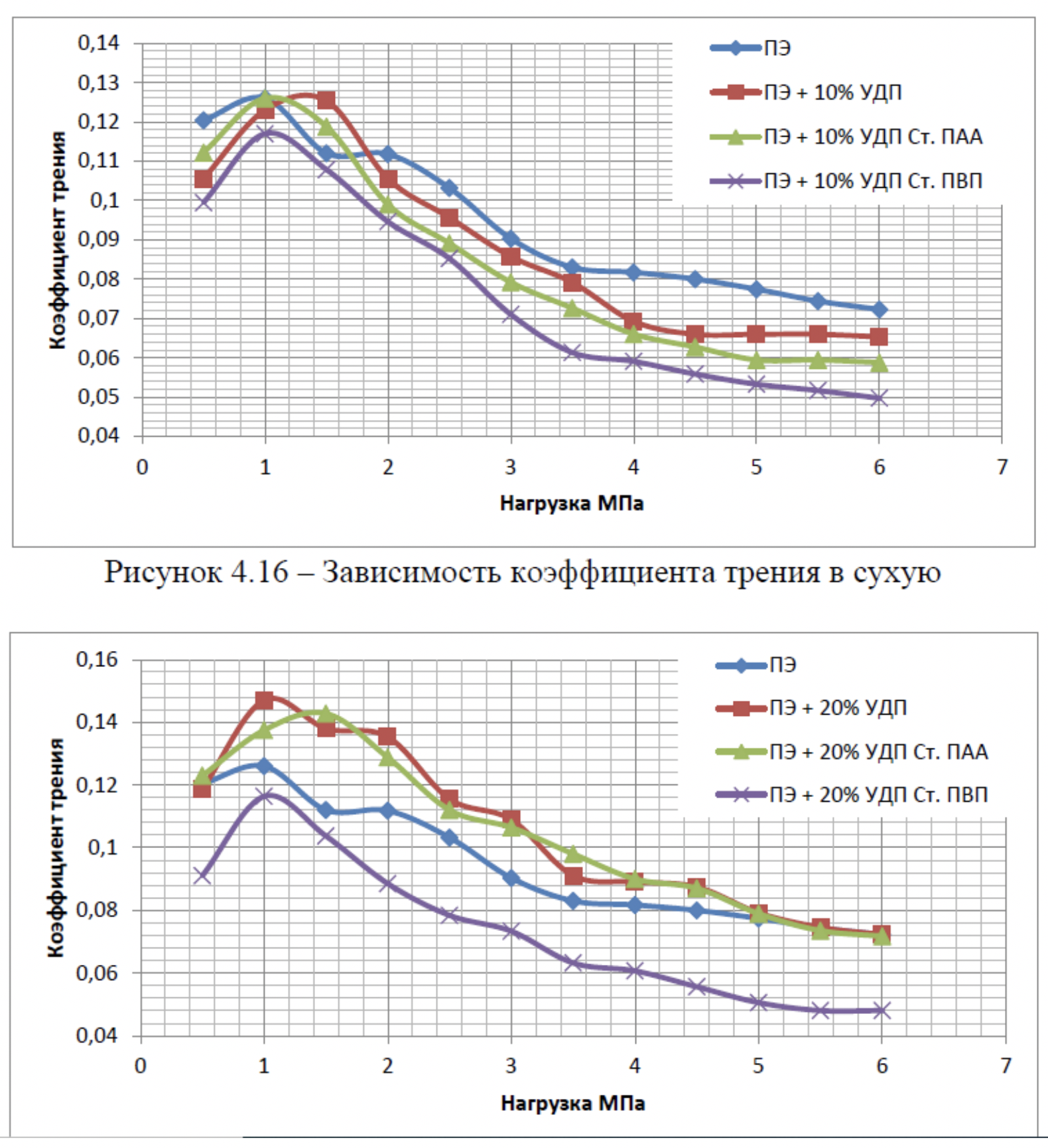
It has been proven that the use of metal nanopowders, modified by water-soluble polymers, as fillers of materials due to the uniform distribution of the filler and the increase in the adhesion interaction, increases the hardness of the materials by 2 to 3 times, the wear resistance by 1,5 , 2 to 2 times and the sliding properties increased by 2,5 to XNUMX times.
Developments that give composite materials a high complex of physical and mechanical properties can be used in highly stressed nodes of friction of machines and mechanisms, instead of expensive anti-friction details made of non-ferrous metal alloys and metal polymers.
The practical results of the dissertation can be recommended for use by research and development institutions and design organizations that are involved in the development, manufacture and implementation of production technologies and composites that use modified ultra-disperse copper powder as filler.
Research methods:
Modern research methods and devices were used to solve the tasks.
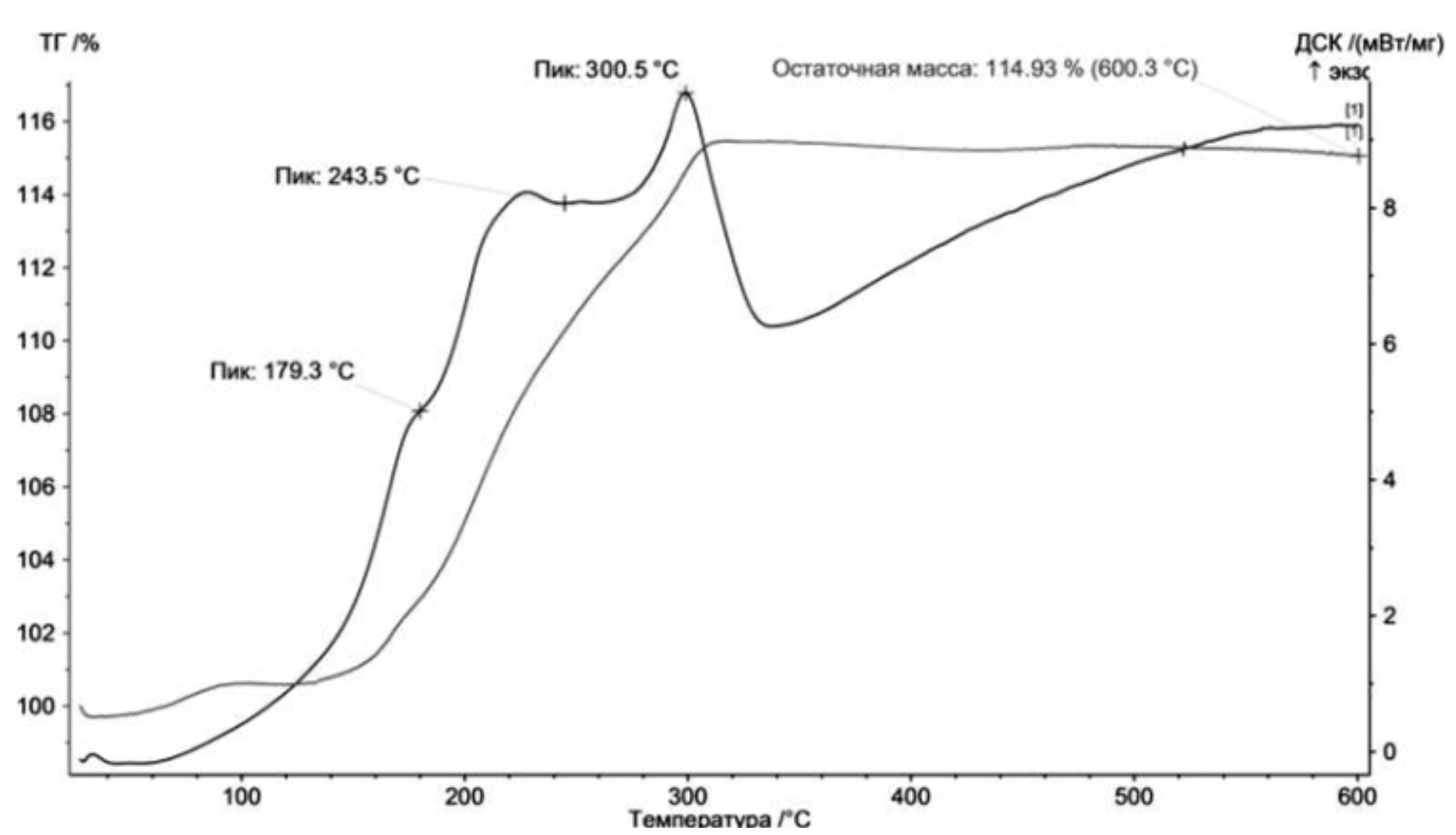
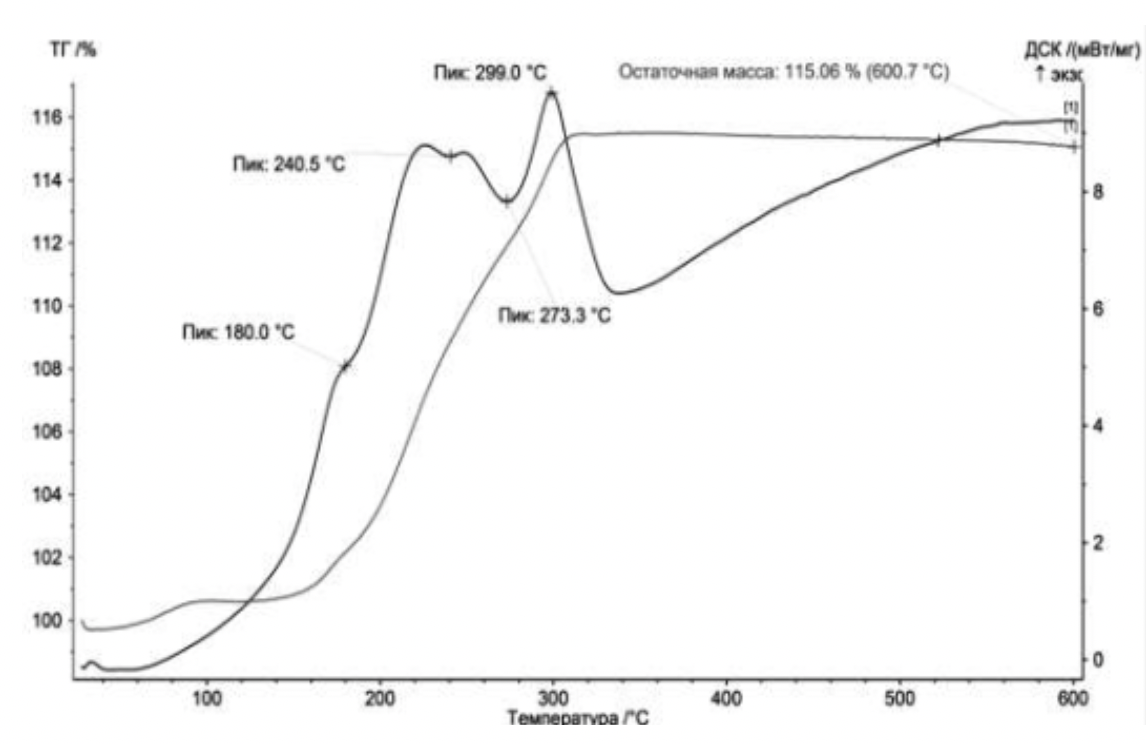
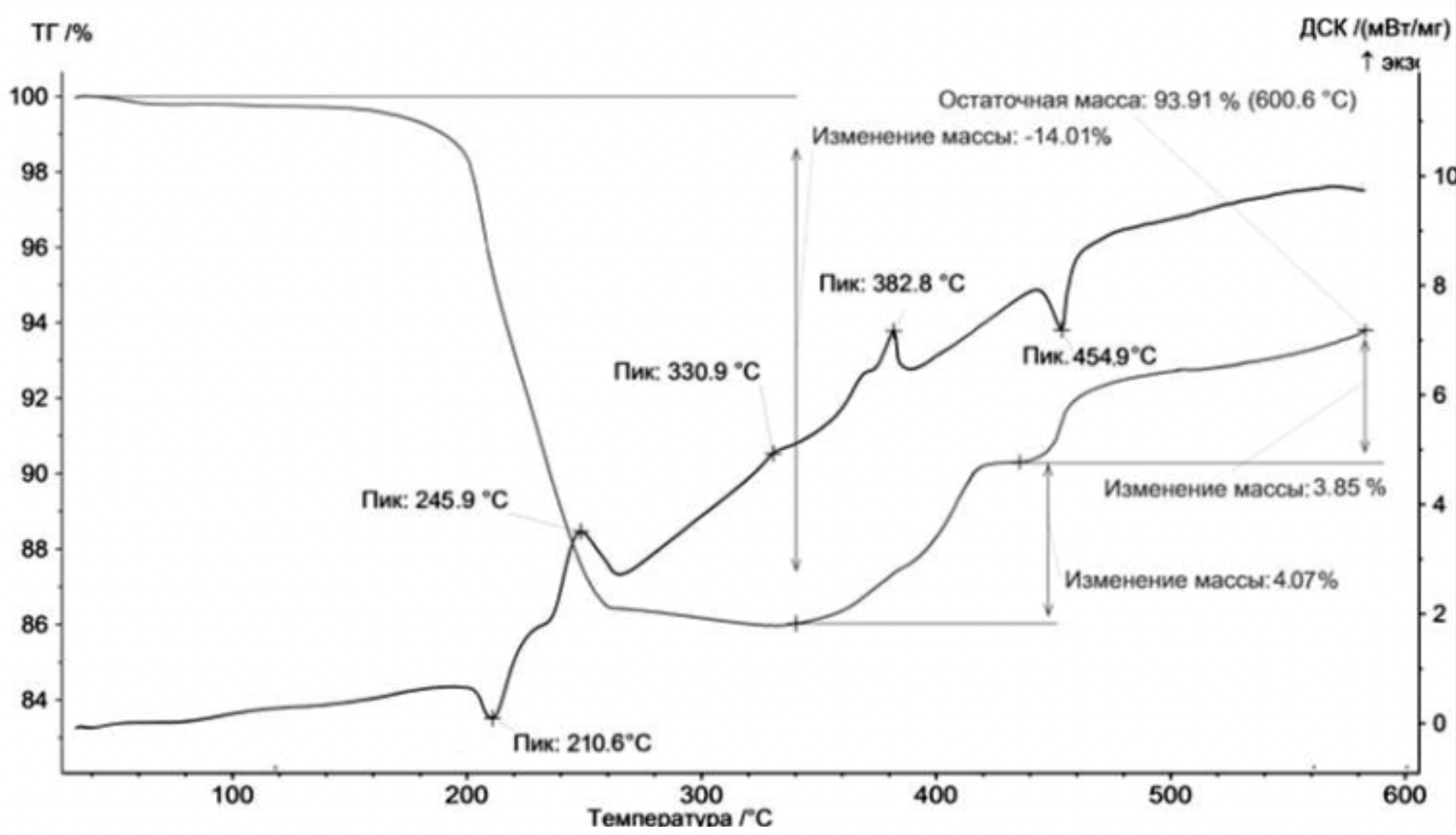
- The thermodynamic analysis was carried out on STA 449C in an oxidizing (air) environment. The samples were heated to 10 ° C at a rate of 600 ° C / min.
- X-ray phase analysis (XRD) was performed with the ARL X'TRA Thermo Fisher Scientific Defractometer. The obtained structure of the ultradisperse copper powder was examined on the energy-dispersive microanalyzer EDAX.
- GENESIS. The investigation of the coordination connections that were formed in the process of extracting the ultradisperse copper powder was carried out by means of infrared spectroscopy on the Varian 640 device. The grain size distribution was determined with the Microtrac S3500.
- The interaction of the filler with the matrix was examined with a Quanta 200 scanning electron microscope.
- The structure of the surface layer of composite materials was examined.
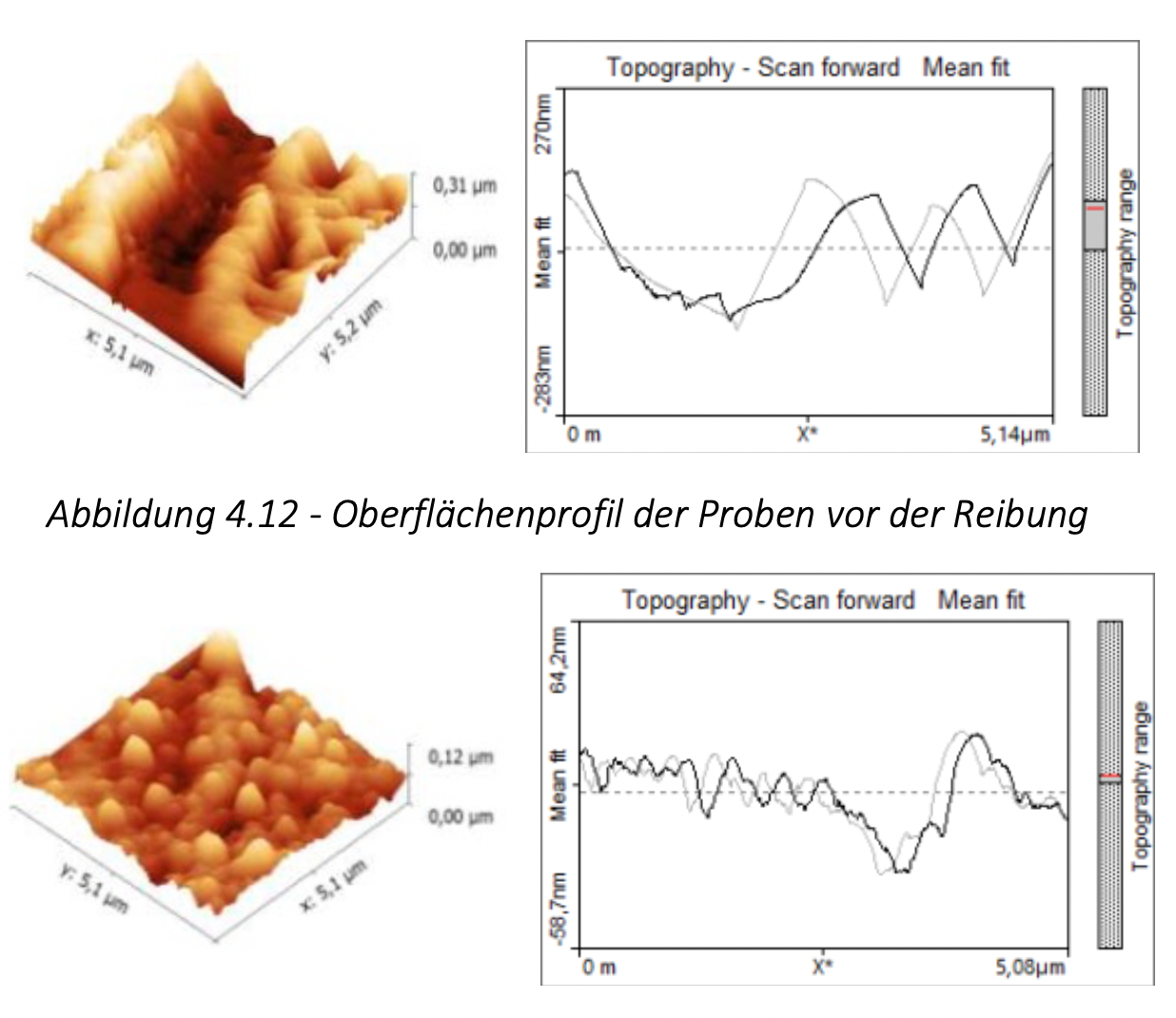
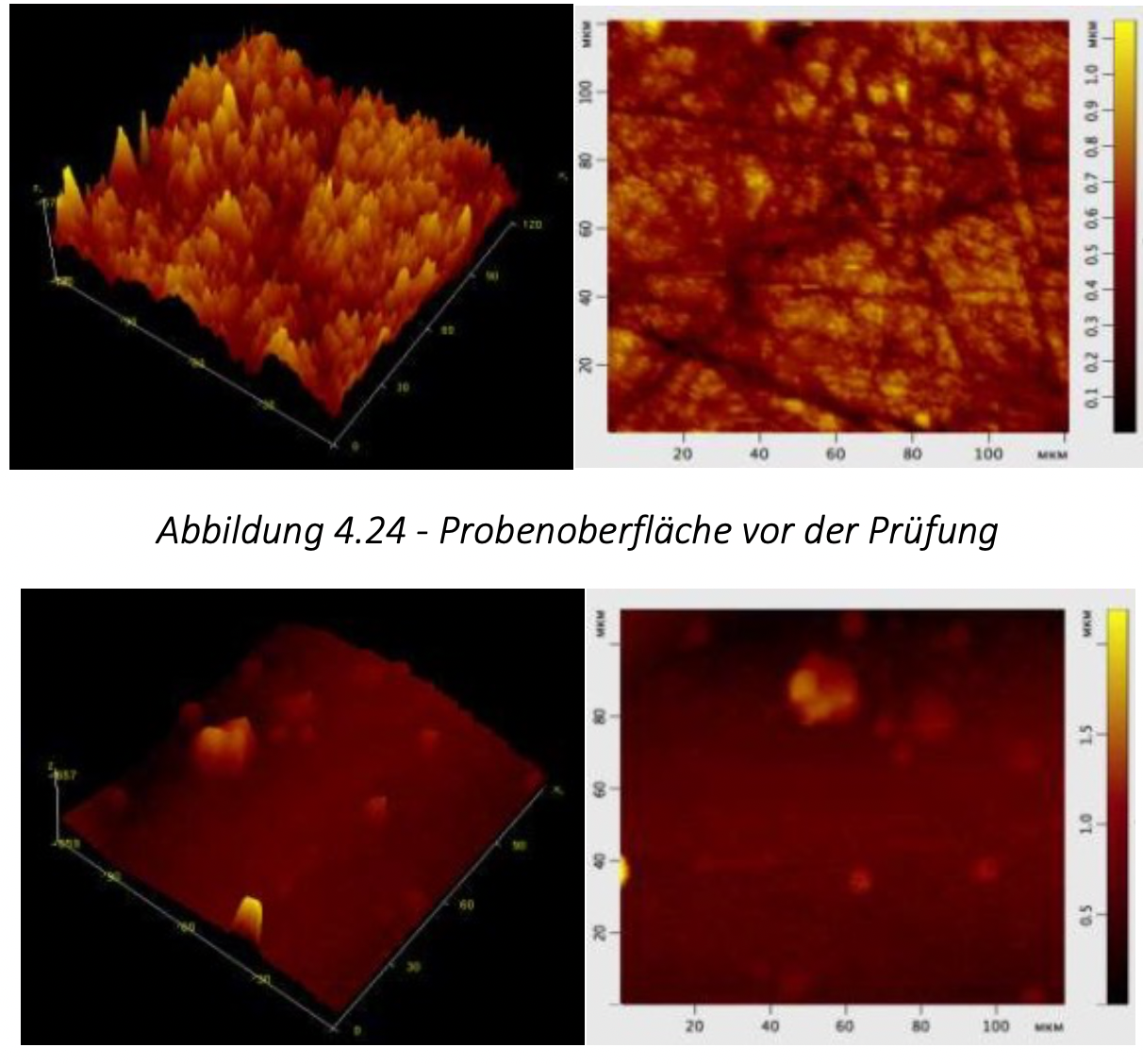
- on a SolverHV scanning probe microscope. The frictional properties of the materials obtained were tested on the TMT-25 final friction machine.
- The linear wear was determined by changing the length dimensions with the help of an optical measuring device with an accuracy of 0,003 mm.
- The hardness of metal-polymer composites was determined on the AS-111 by pressing a ball (according to Brinell) according to GOST 9012-59.
- The definition of the breaking stress during compression was carried out on the device P-0,5 according to GOST4651-82. The results presented in the dissertation do not conflict with experimental and theoretical data from other researchers, which have been published in the free press.
- Technology for the production of copper nanopowders by electrolysis using polyvinylpyrrolidone and polyacrylamide nanoparticles.
- The use of ultra-disperse copper and copper polymer powders, which are obtained from ammonia solutions, as alloy additives to polymer composites enables the increase in hardness and the reduction in wear during friction, reduce the sintering deformation, increase the maximum compressive force.
- The determination of the dependence of the influence of the stabilized properties of Ultradisperse copper powder on the mechanical properties of metal-polymer composites.
- Mathematical model that makes it possible to find the optimal ratio of the concentrations of the ultradisperse copper powder in the polymer matrix, thereby achieving the greatest strength of the material.
Degree of reliability and approval of the results.
The degree of validity of the results is confirmed:
- Agreement of the results with the basic provisions of powder material science as well as agreement of experimental data and scientific knowledge with the generally accepted provisions published in printed publications.
- Application of software for processing experimental research results.
Investigations were carried out on the certified devices.
The powders were tested as alloy additives to composites with fluoroplastic-4 and polyethylene-277 polymer matrix.
The main provisions and results of the research were reported at the annual scientific, technical and research conferences:
- VI International Scientific and Practical Conference “New Materials and Technologies of Their Production”, Novocherkassk (2012), as well as at the
- XV International Scientific and Practical Conference “Curable Coating and Repair Technologies: Theory and Practice”, St. Petersburg (2013).
The author's personal contribution.
All essential results of the dissertation were personally accepted by the author.
The author was directly involved
- when planning your dissertation research,
- in the selection of research objects,
- in the development of technology for the production of ultra-disperse copper powders stabilized with water-soluble polymers, for the production of composite materials made of metallic anti-friction polymers.
The formulation of problems, the selection of research objects and the way in which problems are solved are in the hands of the author.
The topic of the dissertation was suggested by AV Skorikov.
He supervised the thesis scientifically, participated in the discussion and interpretation of the results. Lipkin MS, Danyshina GA and Shishka VG participated in the development of the technology for the production of stabilized ultra-disperse copper powder and technological methods for the production of metal-polymer composites as well as in the creation of a mathematical model.
Publications on the topic of the dissertation.
A total of 9 scientific papers on the topic of the dissertation were published, including
- two magazine articles [78, 119] in published articles recommended by the HIGHER ATTESTATION COMMISSION of the Ministry of Science and Higher Education of the Russian Federation.
A number of papers in the field of international conferences [5, 10, 12, 100 125].
Scope and structure of the work. The work is 135 pages, 62 of which
a drawing and 17 tables. The dissertation consists of an introduction, four chapters, a conclusion and a literature list with 134 titles.
Chapter 1
Analysis of scientific, technical and patent literature
1.1 Applications of ultra-disperse copper powders
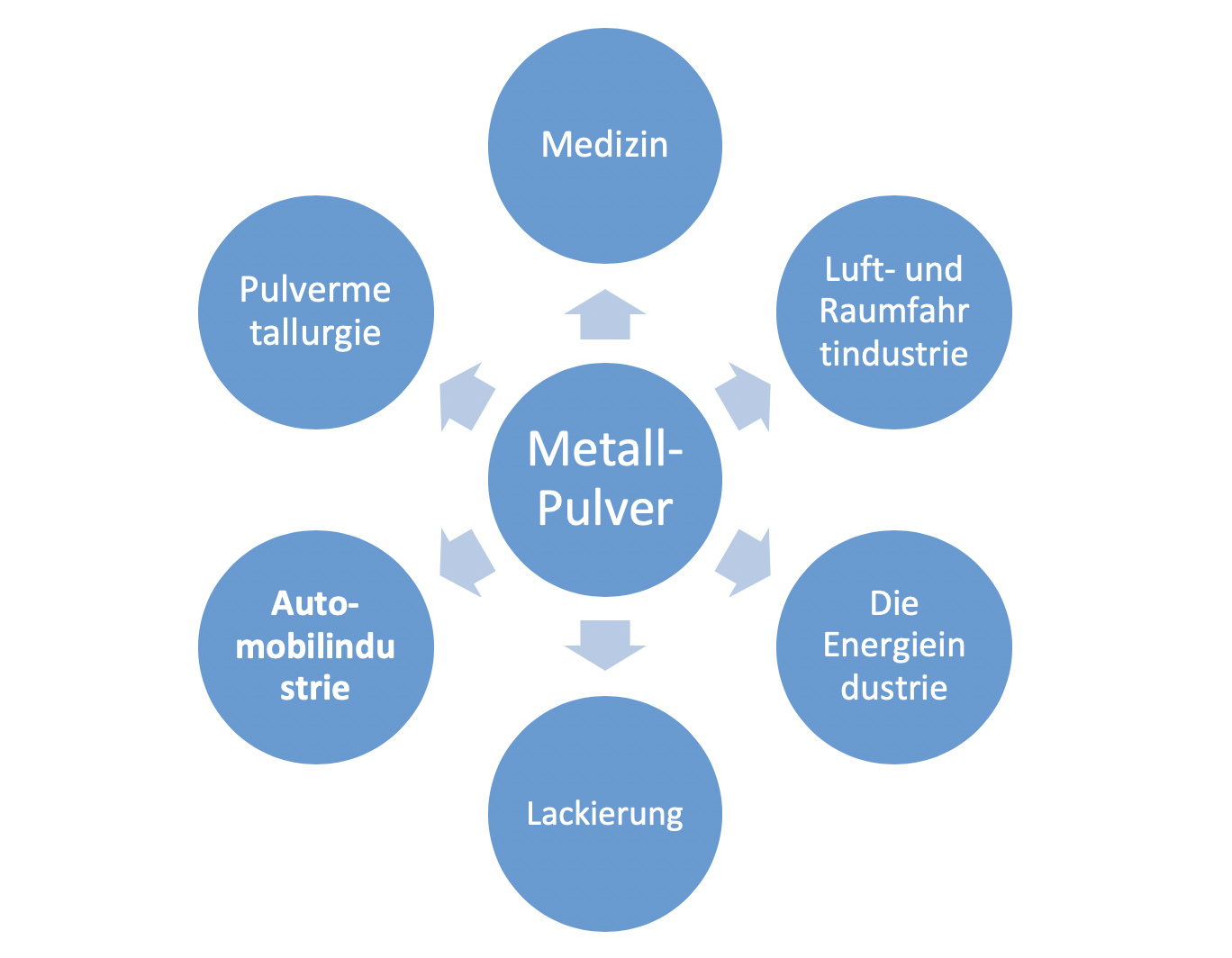
The areas of application for copper powders are currently growing significantly and are no longer restricted to powder metallurgy.
Due to specific properties such as electrical and thermal conductivity, copper powder is actively used in almost all areas of technology and its area of application is constantly being expanded (Figure 1.1).
The widespread use of powders in various areas is linked to their ability to significantly improve the parameters of existing technological processes and to create new technologies.
Ultradisperse copper powders are widely used in powder metallurgy, as biologically active additives, as catalysts in the chemical industry, as additives in paints and other chemical products, such as lacquers, as pigments in printing and packaging, and in many other industries [11-13] ,
Figure 1.1 - Applications of metal powders
The antiseptic properties of ultradisperse copper powders are already known.
The bioactivity studies of unmodified dressings and powder-modified materials [14] showed that the control sample did not inhibit the growth of the Staphylococcus culture.
The material modified with PMS-1 copper powder has practically no zone to inhibit bacterial growth. On the contrary, materials that have been modified with ultra-disperse powders have a strong antimicrobial activity.
The results of the bioactivity in a dense culture medium correlate well with the results of the bioactivity assessment in a liquid culture medium with subsequent sowing.
A probable mechanism of bacterial death is the interaction of copper ions with functional groups of amino acids, proteins of bacteria, which leads to the denaturation of proteins in the cell; Disturbances of the enzymatic balance inside the microorganism; Leakage of soluble vital substances from the cell, which leads to the death of microorganisms.
Ultradisperse copper powders are used in powder metallurgy:
- in the production of electrical contact materials that are used in switchgear, NC and sliding contacts of electrical trains in railways, urban and industrial traffic, and also as automatic electrical switches, points and switches, connectors for arc welding lugs, etc. [15 -16 ];
- at reception of anti-friction materials both with a metal matrix and with polymer, used in various highly loaded nodes of friction [17];
- In the production of construction materials, which are subject to increased requirements in terms of electrical and thermal conductivity, corrosion resistance and decorative appearance. Products made from such materials are used in various areas of science and technology, such as mechanical engineering, shipbuilding, instrument making, and the automotive industry.
In the chemical industry, copper powder is used for the production of catalysts for the complete oxidation of hydrocarbons [22-24].
After considering a wide range of powder applications, it can be concluded that the efficiency, reliability and practicality of powders in various application areas necessitate the development of technologies for the production of ultra-disperse copper powder with the required particle size distribution, chemical purity and particle shape for each specific application.
1.2 Methods for Obtaining Ultradisperse Powder
One of the most important directions in the development of modern technologies is the miniaturization of products for various functional purposes, which leads to savings in the material and energy costs associated with their manufacture and operation, and increases the possible uses in areas where the requirements for size and weight reduction are particularly high are high.
Furthermore, as a result of miniaturization, there are significant qualitative changes in the design parameters and thus the properties of the products created, which opens up fundamentally new ways of practical application.
The development of miniaturization has led to the formation of a group of nanotechnology and the creation of nanomaterials (25).
There are different approaches on how to determine what nanomaterials are. The simplest approach applies to
- with geometric dimensions of the structure of such materials.
- Materials with a characteristic microstructure size of 1 to 100 nm are today called nanostructured [26].
In order to investigate nanomaterials, their atomic structure is first examined, the types of atoms that are building blocks and their mutual position in space determined.
Most of the nanoparticles have a crystalline nanostructure.
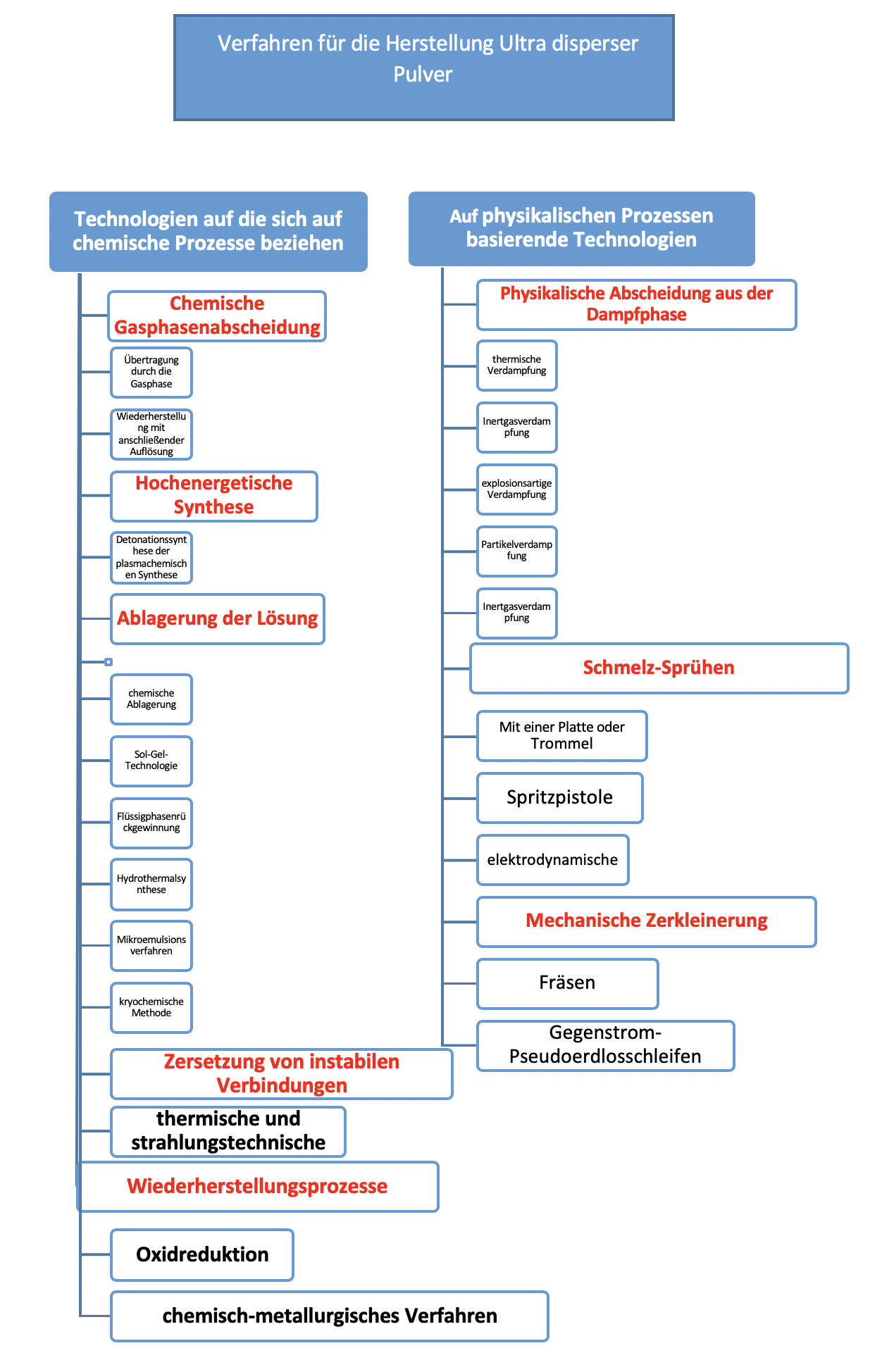
The most widespread technologies for the production of ultra-disperse powders can be roughly divided into two groups (Figure 1.2). The first group includes technologies based on chemical processes, and the second group includes physical processes.
Figure 1.2 - Main Method of Obtaining the Ultra-Disperse Powder
The processes based on physical processes for the production of ultra-disperse powders ensure the conversion of the starting material into a powder without any noticeable change in the chemical composition. The most frequently used methods are the grinding of solids in mills of various designs and the dispersion of melts.
Chemical methods include technological processes for the production of ultra-disperse powders that are associated with the physical and chemical conversion of raw materials.
The resulting kidney connection powder can differ significantly from the original material.
1.2.1 Technologies for the production of ultra-disperse powders based on chemical processes
The authors of the work [27-29] highlight a number of general approaches that are typical of technologies that are based on chemical processes in the production of ultradisperse powders and that differentiate them from ordinary powders:
- high rate of particle nucleation centers,
- low particle growth rate,
- the largest particle size of the resulting particles is not more than 100 nm,
- narrow range of particle size distribution,
- the stability of the extraction of particles of a certain size range,
- the repeatability of the chemical and phase-related composition of the particles,
- Increased demands on the monitoring and control of the incoming process parameters.
Chemical vapor deposition technology
The technologies in this group are based on the use of thermochemical reactions, metal compounds that are sprayed in the reaction chamber and form aerosols. Sprayed salt solutions in a certain zone are thermally decomposed to form solid sediments in the form of ultradisperse powder and gaseous substances or undergo chemical reactions, likewise with the formation of powder and gaseous substances [30].
The most commonly used raw materials are metal halides (mainly chlorides), alkyl compounds, carbonyl, oxychlorides and organometallic compounds.
The size of the resulting particles can be controlled by the temperature and the deposition rate. This technology provides ultra-disperse powders of silicon, boron, titanium oxides, zirconium, aluminum, nitride, silicon carbides and carbonitrides, titanium dioxide and copper with particle sizes from 20 to 600 nm.
Technologies for chemical vapor deposition include high temperature or flame hydrolysis. [31]
It is based on the interaction of compounds, mainly chlorides, in a hydrogen-oxygen flame.
The following chemical reactions (1.1 to 1.3) explain why
This process is also "flame" is called.
N2 + O2 → N2O (1.1)
2CuCl2 + 2 H2 O → 2CuO + 4 HCl (1.2)
In total:
2CuCl2 + 2H2 + O2 → 2CuO + 4NSl
(1.3)
The water created by the interaction of hydrogen and oxygen causes a very rapid and quantitative flow of the CuCl hydrolysis at 1000 ° C.
The only by-product of the reaction - hydrogen chloride - is separated off and fed back into the process for the recovery of CuCl2.
The silicon dioxide (Aerosil) produced by this process consists of units of amorphous primary particles with a spherical shape and a size of 5-10 nm, which are part of secondary particle units with a size of more than 100 nm.
The disadvantages of this method include the high content of oxides and by-products in the resulting EDP and a large variation in the particle size distribution.
Plasmochemical synthesis
This method for the production of ultradisperse powders uses the low temperature plasma of the arc or glow discharge (conventional, high frequency) or ultra high frequency discharge.
Metals, halides or other compounds are used as the starting raw material.
The work [32] offers to obtain composite ultradisperse powders by plasma chemical processes.
This material contains particles that consist of a core and a shell.
The interaction of the plasma with the processed substance ensures the melting, dispersion, evaporation, and then the recovery and synthesis of the product with a particle size of up to 10 nm, including the parameters of the so-called - a critical fetus.
The starting materials are fed to the plasma as a powder.
The following processes take place in the plasma flow: heating of raw material particles to high temperature, their melting, evaporation, chemical reactions, formation of product particles, cooling.
The authors of the work [33] have developed a universal method for the production of ultradisperse powders from metals, alloys and compounds - recovery and synthesis in chemically active plasma.
The ultradisperse powder obtained in this way has a relatively low dispersion. The particle shape is almost spherical. Because of the high plasma temperature and high speeds.
With this method, it is possible to obtain ultra-disperse powders of various metals and alloys.
This is due to the transition of almost all starting materials into the gaseous state with their subsequent condensation in the form of ultra-disperse powder with particles of regular shape, with sizes from 10 to 200 nm.
The highest temperatures and powers are achieved through the use of arc plasma cartridges, and the cleanest and most homogeneous ultradisperse powders are obtained with microwave 20 ultra-high frequency plasma cartridges, use a gas discharge device to obtain a low temperature plasma.
The advantage of this method is that it ensures the production of products with the required chemical composition, physical state and shape dimensions, also in the form of ultra-disperse powders.
The disadvantages of this method are:
1) Sufficiently large dispersion for oxides and complex compositions;
2) High corrosive activity
volatile compounds, high adsorptive surface energy of nanoparticles,
which lead to the adsorption of synthesis by-products on their surface which are difficult to remove; 3) the need for expensive equipment
Solution separation technologies
What this group has in common is the implementation of chemical reactions in aqueous salt solutions. Several different methods are used [34-36] based on the use of water or organic substances dissolved in water.
Metal salt solvent with chemically active substances used as ultra-disperse powder non-solvent.
This technology is similar to chemical metallization technology, except that it is not activated. Particle emission occurs on the surface over the entire volume of the solution.
Powders of copper oxide with a high degree of purity, homogeneity and dispersion can be obtained by extracting copper salts (copper salt N, N'-dinitrourea) from the solution in the presence of an organic solvent such as dimethyl sulfoxide.
The solution becomes 1-6 within 110-150 hours oC is heated and copper oxide powder is released from the suspension [37].
The process for the recovery of copper powder by recovery from copper salt in the presence of the reducing agent is considered in [38].
Copper sulfite is used as a salt and glucose as a reducing agent.
The average size of the resulting copper powder is 35-45 nm, and the gain up to 90%.
In the chemical separation from the solutions of the salts, you add a substance - the separator and carry out the separation of the metal oxide powder.
The precipitation conditions are regulated by changing the pH value, temperature and adding buffer solutions.
Ammonia solutions, ammonium carbon dioxide, oxalic acid, ammonium oxalate are most often used as precipitants, and soluble nitric acid salts are preferably used as precipitants.
In addition, this method has found sufficient application to obtain multi-component, ultra-disperse composite powders when several compounds are precipitated from multi-component solutions [39,40].
The complexity of this method lies in a practically uncontrollable process of extraction, which makes the extraction of powders with a particle size of less than 0,5 μm almost impossible.
The main disadvantage of the method is the use of large amounts of solutions and the difficulty of their disposal, a considerable content of impurities in powders and a large dispersion of the particle size.
The authors of the work [69] propose a method for obtaining ultra-disperse copper powder by mixing five-period copper sulfate with glycerin.
With the subsequent heating up to the complete dissolution of the components, then organic acid (formic acid or oxalic acid) is introduced as the initiator of the copper extraction and after washing the ultradisperse copper powder is treated with pentane or a stearic acid-alcohol solution with subsequent drying.
The disadvantage of this method is a complex copper recovery process, high reagent consumption and high temperatures.
One of the methods that consists in reducing ultradisperse particles from solutions is the liquid phase reduction method. With this method only ultradisperse powders of metals with low values of the reduction potential (copper, silver, nickel) are obtained [41]. It consists in the preparation of an organic metal salt solution, followed by the addition of a strong reducing agent and the separation of the deposited ultradisperse metal powder.
The particle size of the resulting powder is 20-40 nm and the particle size distribution is very small due to the increased viscosity of many organic solutions.
An example of the application of this method is the production of ultra-disperse copper powder [42] when using an aqueous solution of hydrazine hydrate with lithium sulfate and a solution of copper nitrate in 4-methylpentanol. These solutions are mixed and obtained
Emulsion, after layering of which the ultra-disperse copper powders are in the organic phase.
The complexity of this method lies in the separation of the powder from organic compounds, which are adsorbed on the surface of ultradisperse powder due to the high surface energy and can have a stabilizing and protective effect. The disadvantage of this method is the low productivity, large losses of ultra-disperse powders when separating and cleaning the powder from organic substances.
Method for hydrothermal synthesis
This method is based on chemical reactions of hydrothermal decomposition and oxidation that take place in aqueous media at high temperatures.
Temperatures (100-370оС) and pressure (up to 100 MPa).
The essence of the hydrothermal method is the heating of salts, metal oxides or hydroxides as a solution or suspension at elevated temperature (usually up to 3000oC) and pressure (approx. 100 MPa). In this case, in solution or the colloidal system undergoes chemical reactions that lead to the formation of a simple or complex oxide reaction product.
The hydrothermal synthesis is carried out in autoclaves, often lined with Teflon, volume 50-300 ml.
The high pressure increases the boiling point, so that the process is carried out at higher temperatures than in aqueous solutions at atmospheric pressure. [43-44].
With this method it is possible to obtain ultra-disperse oxide powders with a narrow grain size distribution.
The disadvantage of the method is the high cost and complexity of the equipment, as well as the frequency of the synthesis process (which can take up to 24 hours) and consequently the low productivity.
Synthesis under the influence of microwave radiation
Synthesis of ultradisperse powders under the influence of microwave radiation is a new and very rapidly developing, promising method.
As with all solution processes, a deposition reaction of the product from the starting solution is carried out, but this is influenced by microwave radiation.
The microwave energy is transferred to the starting material, which leads to its rapid heating, which initiates a chemical interaction.
The mechanism of the microwave effect on the synthesis of ultradisperse powders has not yet been fully clarified.
The extraction of some simple and complex oxides by this method is described in the literature [45].
Technology for the decomposition of unstable connections
This technology is currently considered a promising way to manufacture ultradisperse powders with particle sizes of 20-300 nm.
The thermal decomposition of azides, oxalates, perchlorates, styphnates, permanganates, carbonates, hydrates, citrates, acetates, hydroxides, alcohols has been most studied [46,47].
The authors [49] propose to obtain ultradisperse metal powders by decomposing metal carbonyl with an induction melting torch.
This method offers high productivity in the extraction of particles and does not introduce contaminants into ultradisperse powders.
The process involves three reactions: thermolysis, oxidation and hydrolysis.
The advantages of this method include the low process temperature, small reaction volumes, no time-consuming and inefficient washing and filtering of the end products, adjustable dispersibility, good sintering properties and high purity of the powders produced.
The disadvantage of the considered method is the complexity of the control and regulation of the particle sizes with simultaneous competing course of two processes - decomposition of the starting compound and sintering of the particles of the end product under the influence of temperature.
All the more reason that the powders produced by this process are very reactive.
Process for electrochemical reduction
Of the generally known methods of synthesizing ultradisperse metal powders, the electrochemical methods [50-54], which, at the expense of the variations in the electrolysis conditions, especially the current density and the electrode potential, enable the speed of electrode reactions and thus the productivity, chemical composition, Check the size and shape of the resulting products.
The versatility of the raw material base, which includes compact metals, alloys, oxides, salts, including metal-containing materials that are to be recycled in the form of scrap, electrode materials from used batteries, industrial waste water and solutions, opens up broad perspectives for the application of electrochemical technologies for the production of ultra-disperse metal powders ,
In addition, the electrochemical processing of waste into highly effective tribological functional materials while at the same time guaranteeing technical efficiency, economy and environmental safety is fully in line with the principles of green tribology.
The authors suggest the extraction of copper powder from ammonia waste by electrophoresis [55]. The particle size of the resulting ultra-disperse copper powder exceeds 300 nm.
The advantage of this method is the use of waste from the radio electronics industry as a raw material, which brings additional economic and ecological advantages.
However, the extraction of particles smaller than 100 nm with this method presents a number of challenges.
In [56, 57] the method for obtaining ultradisperse metal powders from the sulfate electrolyte with soluble anode.
The sediment that forms on the cathode during the electrodeposition can be both a loose and a dense layer of many microcrystallites.
Many factors influence the sludge texture, such as the type of substance and solvent, the type and concentration of the ions of the target product and the foreign substances, the adhesive properties of the precipitated particles, the anode and cathode current density, the ambient temperature, the diffusion conditions and others. The main advantages of the method are experimental availability, multiple use of electrolytes, working with secondary materials and the ability to control and monitor the process of obtaining ultra-disperse powders.
The disadvantages of the method are side reactions, which take place in the solution with the formation of precipitates and contaminate the resulting powders, and lead to passivation of the electrodes, which significantly reduces the productivity of the system.
Study of work [27-57] which, with the development of methods for the production of ultradisperse powders by chemical methods, showed that most ultradisperse powders have a high surface energy and the attempt to reduce them therefore the pronounced tendency for integration into Aggregates and agglomerates.
All of this makes it necessary to consider not only the size of the individual particles, but also the size of their connections.
In the case of aggregates, the bond between the crystals is assumed to be stronger and the intergranular porosity is lower. In the subsequent compression, aggregated powders require higher temperatures and / or pressure than unaggregated powders in order to achieve the desired porosity of the material.
1.2.2 Technologies for obtaining ultradisperse powders based on physical processes.
Methods based on physical processes ensure the conversion of the starting material into a powder without any noticeable change in the chemical composition.
The most frequently used methods are the grinding of solid materials in mills of various designs and melt dispersion.
Such methods include technological processes of powder production that are associated with physical transformations of the raw materials.
The chemical structure of the powder obtained differs significantly from that of a starting material.
Atomization of the melt
This group of methods is based on the rapid spraying and cooling of the melt of the starting material.
The technology of powder production with a particle size of at least 58,59 nm is described in [100].
At the same time, the resulting powders with particle sizes of 0,5-10 µm have a crystal structure and can therefore be classified as nanomaterials and their technology can be related to nanotechnology. The powder production with this method can be carried out in a protective atmosphere. The following three variants of this technology are currently mainly used for the production of nanocrystalline powders. Contact cooling with a water-cooled disc or drum. The authors of the work [60] use a rapidly rotating water-cooled disc or drum, to which the molten material is fed.
The pane material is selected so that a high thermal conductivity is guaranteed.
As a rule, copper is used as such material.
The cooling rate of up to 108 K / s is achieved. The surface of the drum or disc is rough (serrated).
In the case of a smooth surface, it is possible to obtain foils, tapes or wires with a thickness of the order of 10-50 microns with an amorphous or crystalline structure.
The resulting powder has a flaky particle shape.
This particle shape can lead to an inhomogeneous structure and anisotropy of the properties in products formed from such powders.
As a rule, the powders obtained by the process under consideration are additionally mechanically comminuted. This is the main disadvantage of the method.
Methods of physical condensation
The most common physical methods used to manufacture ultradisperse metal powder, in fact, they are dispersion condensation because the first stage is the dispersion of the metal to atomic dimensions (evaporation), and then the condensation.
Particularly noteworthy is the method for obtaining ultradisperse composite powders, which involves heating the substance through a rhetorical beam of electrodes at atmospheric pressure up to the vapor phase state,
Condensation by cooling the vapors in the gas stream and separating the resulting two-phase system.
In this case, two single-element substances are heated, whereby particles of solid, ultra-disperse composite powder of the core-shell type are formed [61].
The manufacture of nanofibers has been much less studied than ultradisperse powder because it is not yet widely used.
One of the most investigated methods for obtaining nanofibers is the interaction in the gas phase with subsequent condensation of the product on a solid substrate.
Essentially, this method can be referred to as the method of evaporation (metal) - condensation (oxide).
Metal evaporates in some way and its vapors react with oxygen. or another gas at high temperature, the reaction product then condenses on a solid substrate [62,63].
Mechanical crushing
Mechanical comminution of material particles is one of the most common methods for the production of powders.
Powders based on brittle materials are particularly easy to produce.
Plastic powder, high-strength and amorphous materials are more difficult to obtain.
In this case, the risk of excessive heating of the material and its contamination with wear products from working parts of the technological systems increases [64].
For mechanical grinding with mills:
the reduction in the material grain size is the result of intensive grinding between the control elements of the mill.
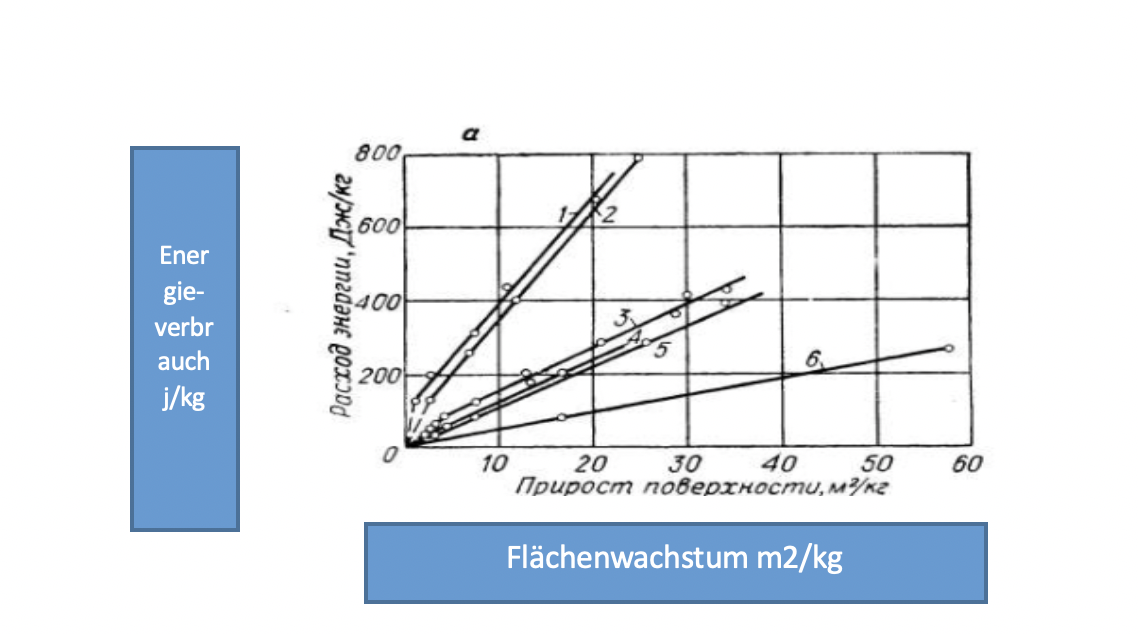
In Rittinger's theory, the energy used for grinding is directly proportional to the newly formed surface.
When comminuting materials such as copper, bronze, aluminum, it has been confirmed that in coarse and fine grinding according to Rittinger's theory, the newly formed surface is directly proportional, proportional to the energy used for grinding or, equivalent to a ball mill, for grinding time [65].
Figure 1.3 shows the dependence for some materials on the surface growth of the material ΔS (and thus to reduce the average particle size) on the duration of the grinding process during the fine grinding of the material in the batch mill.
Figure 1.3 - Dependency of surface growth during grinding
- Silicon oxide; 2 - slate; 3 - anthracite; 4 - barium t; 5 - resin charcoal; 6 brown coal.
The average particle size achieved by mechanical grinding of powders can be in the range of 30 nm. High-energy mills (attritors and simoloyers) with a fixed body drum and agitators, which transmit the movement of the balls in the drum, are used for the comminution of metals [66-68].
In this case the ground material is mainly the result of abrasion and not of impact. The main disadvantage of the process is contamination of the powder by wear on the working parts. In the countercurrent grinding process in the fluidized bed, the powder particles are crushed by collision with one another. The processes of mutual collision of particles accelerated to high speeds in the gas jet take place in the middle of the fluidized bed, which are formed by these particles.
Only a very small part of the particles come into contact with the walls of the chamber in which the grinding process is carried out.
The particles are removed by an inert gas flow from the grinding zone into the upper part of the system, which is equipped with a separator for particle size separation. Particles that are smaller than a certain size are transported with the gas stream to the filter system, where they are separated from the gas stream and passed into the storage funnel. The coarse particles are returned to the grinding zone by the separator.
1.3 Methods of ultradisperse powder stabilization
One of the main problems in the development of metal-polymer composites with ultradisperse powders as fillers for metals is their high surface energy, which leads to their agglomeration and uneven distribution in the matrix.
The composite materials obtained in this way not only lose the properties expected from the introduction of ultradisperse powders, but are also heterogeneous materials with deteriorated physical and mechanical properties. This explains the growing interest in methods for stabilizing ultradisperse powders.
Various methods for stabilizing ultradisperse powders are known from the literature [69-73], including the use of various enveloping (encapsulating) substances, passivation, stabilization by colloidal methods. In order to prevent the agglomeration of particles, the author of the work [74] uses surfactants.
This method is based on processing the resulting ultradisperse powder with solutions of various surfactants to reduce the surface energy. However, it is not always possible to use this method of maintaining structure; furthermore, surfactants cannot significantly increase the shelf life of ultradisperse powders and are difficult to remove from the particle surface. In dilute suspensions, agglomeration can be prevented by electrostatic repulsion [75].
Controlled injection
The electrolyte creates an electrical double layer and agglomeration is prevented if the electrostatic repulsive forces exceed that of Van der Walsow gravity.
The result is ultra-disperse oxide powders. If necessary, ultra-disperse metal powders can be produced by heat treatment in a reducing medium. The authors [76] deal with the question of stabilizing ultradisperse powders with organic pigments, polyglycols, gelatin, polyacrylates of sodium or potassium. The stabilizer is made as a solution in distilled water.
The extraction of ultradisperse powders into the resulting medium takes place in atomic and / or ionic form by chemical or electrochemical reactions with the formation of metal particles. The particles surrounded by layers of stabilizer molecules retain their properties in the water / stabilizer / particle system for at least 12 months.
It is known that ultra-disperse aluminum powders are widely used in compositions for the production of composite materials [77-78]. Ultra-dispersed powders, which are obtained in the process of electrical explosion of wire, are highly agglomerated.
Avoiding agglomerations is possible by forming a particle shell with a higher melting point when the particles themselves are formed.
The work [72] showed that it is possible to reduce the agglomeration of aluminum particles by the formation of Al2O3 shells on the surface of the particles in the process of powder production. Carbon or aluminum carbide can be used to reduce the amount of Al2O3 in the nanoparticles.
Aluminum powder is also obtained in an inert, methane-containing environment by using a micro-arc discharge.
The surface of the individual particles forms a carbon shell, but the majority of the particles are in the carbon matrix, which means that the mixture cannot be processed later.
In addition, the resulting powder does not contain aluminum carbide, its absence can be explained by the rapid cooling of the aluminum particles.
In an electrical explosion, wires in a carbon-containing environment create conditions for the formation of carbide particles: aluminum particles that form after condensation have a high (over 2000oC) temperature and its cooling takes place in a gas environment.
The work [79] presents studies on the possible separation of ultradisperse powder particles from agglomerate components using ultrasound in alcohol.
The particle size of the resulting ultradisperse powder is 10 nm.
The authors of the work [80, 81] suggest using both mechanical stirring and ultrasound treatment to stabilize ultradisperse powders. This ensures the sedimentation stability of the suspension with highly dispersed particles. In order to obtain highly ordered silver nanoparticles with a ligand shell, 3-6 mol / g sodium oleate and 10 mol / g sodium borohydride are added to a highly viscous aqueous solution made from polyvinyl alcohol or gelatin.
The reaction takes place without stirring. You get particles with a ligand shell and a low degree of aggregation [82]. The method for obtaining metal polymer particles is proposed in the literature [83].
Composite polymer material based on ultra-disperse silver powder, which is used as a stabilizer of carboxymethylchitin particles with a concentration of 2-4% by mass.
Also of interest is the method described in [84] for the production of copper powder with an increased content of nanofraction. In order to achieve the goal, the authors proposed an optimized electrolysis process, given the significant influence on the size of the copper sediment particles, concentrations of the main components of the sulfate electrolyte.
In order to reduce the average particle size of the powder in the electrolyte, there are also functional additives, namely gelatin and polyethylene glycol. According to the research results, the most effective way was to introduce gelatin into the electrolyte.
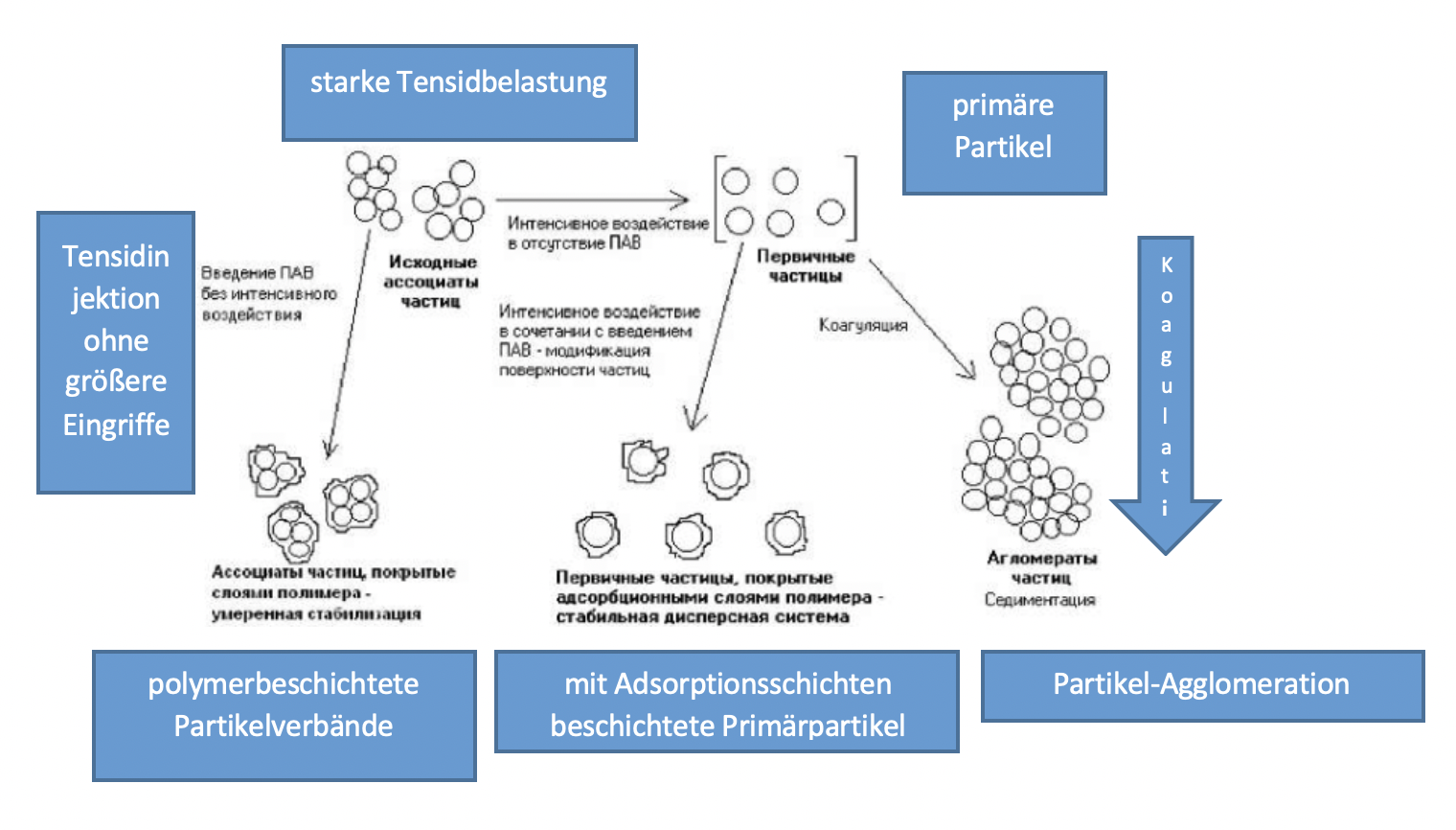
The main task of stabilizers is to achieve the highest possible dispersion of ultra-disperse powders. In the work [85] it was found that the activity of nanoparticles increases with the surface of the medium, which contains a polymeric modifier and a solid phase.
The smaller the particle size of ultradisperse powder, the more contact points it has with the polymer and the more intense its interaction.
It can be assumed that the role of the polymer in combination with intensive mechanical influencing of the water systems of pigments and fillers is the adsorption on the surface of the particles with the formation of protective layers,
and mechanical activation deagglomerates the associations and activates the primary particle surface.
This can be illustrated by the following points.
Figure 1.4 - Effects of intensive mechanical influences on the dispersion of aqueous pigment and filler systems in the presence and in the absence of polymeric stabilizers
In the absence of polymer stabilizers, mechano-activation leads to particle enlargement, the particle associations in the process of mechano-activation are dispersed in primary particles with an activated surface, which quickly coagulate and become agglomerates.
In the presence of the polymer, the dispersion process is “fixed” by the formation of protective films. Thanks to the polypyrrh hole layer, it is possible to protect copper, nickel, iron powder, etc. from oxidation by atmospheric oxygen.
The method is practical in that no assignment from the dispersion is necessary to protect against oxidation.
The protective layer is electrically conductive, but does not block the magnetic properties of the particles and combines well with the biological environment. [86,87].
The process for the production of ultradisperse metal powders [88], including the production of direct micellar dispersion of a reducing agent based on an aqueous solution. Cationic surfactants with halogen counterions and ion recovery of metals in a pure micelle system, which differs in that the recovery is carried out with hydrophilic addition of organic acid, preparation of a micellar solution of a surfactant with salt of the metal obtained and reduction of the metal ions by combining these two solutions carried out when stirred to obtain the dispersion of metal particles obtained after the recovery of ultradisperse powders protect the polypyrrole coating from polymerization Pyrrloch salt by acidifying the above-mentioned dispersion of ultradisperse metal powders with mineral acid, adding pyrrloch, hydrogen peroxide and mixing.
Nowadays, the method proposed by the authors of [89] for obtaining dispersion of nanoscale metal powders is well known, which involves an oxidation-reduction reaction of the corresponding metal formation in the vicinity of hydrocarbons with the addition of sulfur-containing surfactants under the action of ultrasonic vibration energy, characterized by the use of alkyliol, dialkyl sulfides, dialkyl disulfides, dialkyl tiocarbamates or alkylthiophenol as sulfur surfactants, surfactants being added in amounts which are determined on the basis of the formation of nanoparticles, to a lesser extent the monomolecular layer.
Also widely known are the methods [90-93] of obtaining ultradisperse copper powder in aqueous media, and distilled water is used as a solvent, and various organic and inorganic stabilizing components and various organic and inorganic stabilizing components are used as stabilizing components Stabilizing components used.
Organic stabilizing components can be polyglycols, polyvinylpyrrolidone, polyacrylates of potassium, sodium, gelatin, inorganic stabilizing components - ammonium citrate, potassium, sodium.
In addition, the powders are treated with 0,05-0,10% stearic acid solutions and 1-2% hydroquinone solution in order to modify them against oxygen oxidation in the air and to increase their stability.
The powders obtained are characterized by good long-term stability over several months.
The authors of the work [94] as a liquid for dissolving salt were selected a water-soluble complex based on various amine derivatives with antiseptic properties. The solution was dispersed by ultrasonic treatment at an elevated temperature. During dispersion, the salt shell is removed by the simultaneous formation of the organic shell on the surface metal particles, which ensures their stabilization.
Examination of the suspension after 100 days of aging at room temperature showed that all properties of the suspension remained stable.
1.4 Metal-polymer composite
The materials reinforced with ultradisperse powders are obtained by methods of powder metallurgy, which are controlled by crystallization, intensive plastic deformation, plasma chemical, detonative, mechanical, self-propagating high-temperature synthesis, pyrolysis, electroshock, electrolysis and others. To date, a considerable amount of experimental data on nanomaterials has been collected [95-98].
However, it is difficult to obtain materials for several reasons. The materials obtained are usually thermodynamic non-equilibrium systems with a defective structure and superfluous surface energy, make the nanoparticles sticky and aggregate. In addition, nanoparticles are chemically active and often lose their unique properties when interacting with other substances.
In the field of the production of composite materials with a polymer matrix, methodical approaches and technologies for the extraction of new nanostructured metal-containing composite materials have been developed, both through the introduction of ultra-disperse fillers and through the extraction of nanoparticles directly in the polymer and oligomer matrix [99].
The modification of polymers with ultradisperse or nanodisperse compounds ensures maximum structuring of the polymer matrix at various levels and the achievement of materials with unique mechanical, electrical, optical and other properties that are often unattainable for traditional composite materials.
When absorbing polymeric composites, the thermolysis of metal-containing monomers, the electrodeposition of metal in a porous structure, the polymerization and the polycondensation can be used in certain modes that enable the uptake of nanoscale particles or clusters directly in a polymeric matrix [ 100].
Ultradisperse powders of metals and their oxides are created by molecular dispersion (atomization or reduction) with subsequent condensation of atomic metal in nanoparticles or evaporation in atomic metal plasma on thin polymer substrates, electrolytic deposition of metals in the nanoporous structure of polymer matrices [101, 102].
At the same time, their thermophysical and physico-mechanical properties improve:
The proof stress increases and its heat resistance decreases by 20% linear coefficient of thermal expansion.
The production of new nanomaterials based on polymers that are difficult to process (fluoroplastics, ultra-high-molecular polyethylene, polyimides, aromatic polyamides, polyesters) is possible through an activation treatment for structural modification of component assemblies.
It promotes the formation of nanostructured materials through the simultaneous arrangement of the initial phases in the nano range, chemical interaction of the components at the atomic level through mechanical-chemical transformations, improvement of their adhesion interaction [103].
1.4.1 Technologies for the production of metal-filled composites with fluoroplastic matrix
The processing and manufacture of filled composite materials based on F-4 is mainly carried out by powder metallurgical processes and differs significantly from the manufacture of composite materials based on other thermoplastic polymers, such as PE, which due to the high viscosity of the polymer even at melting temperatures as well as Inertness is caused, which does not allow a high interaction of the filler components with the matrix to be realized [104].
The technological process of manufacturing metal-filled fluoroplastic composite materials consists of the following successive stages: preparation of the components; Preparation of the compound mixture; Press; Sintering the presses; Calibration. Mechanical mixtures of powder F-4 and dispersed reinforcement phase are used in the production of composite materials as the starting material.
There are two main ways to get blends of fluoroplastic composites:
1) Mixing and grinding powder F-4 and filler on mechanical stirrers and mills [105];
2) Coagulation of the F-4D suspension together with the filler [106].
In the first case, the best results are obtained by mixing at low temperatures (cooling with liquid nitrogen) or in shock mode, since F-4, which has a fibrous structure, sticks together easily at normal temperature and prevents the even distribution of the filler [107].
Typically, the main method for obtaining filled fluoroplastic composite materials is to mix F-4 powder with fillers and then melt the molded system.
F-4 is characterized by a high melt viscosity, it is very difficult to achieve a good homogeneity of the system in this way and to prevent agglomeration of dispersed fillers.
For this reason, other approaches have been developed to produce nanocomposites for F-4 and other thermoplastic polymers.
A method for treating ultradisperse powders with substances with reduced surface energy has been proposed.
Ultradisperse powders, when mixed with polymers and metal powders in planetary mills, produce inorganic materials with evenly distributed filler throughout the volume of the polymer. Such activation, however, greatly changes the starting particles of the metal powder and transforms them into capsules of 10-70 nm, which are coated with a thin polymer layer with low surface energy.
Such particles are best suited for fixation in the matrix, while at the same time the particle shape is optimal with regard to the particle interaction with a polymer surface.
Therefore, the use of methods that exclude the deformation of composite particles is the most optimal for the production of composite nanomaterials [108-111].
Differences in the results of [112-114] research on micro- and nanocomposite materials show that as the size of the elements of composite materials approaches the nano-level, the reactivity of F-4 increases and its influence on the properties of other components of composite materials increases ,
The observed effects can be used to produce new, promising materials.
The introduction of mechanically activated ultra-disperse ceramic particles leads to the formation of heat-resistant polymers and in particular in an F-4 mesh structure on the friction surface.
This layer serves as a protective shield to localize contact deformation and to protect the material from wear.
Developed anti-friction materials based on F-4 and activated synthetic ultradisperse ceramics, natural fillers (zeolites, diamond waste) are characterized by increased wear resistance (100-370 times) and deformation resistance properties (by 20-30%) compared to the original polymer [115].
The manufacture of metal-polymer nanocomposites is therefore promising, as it enables materials with high and sometimes unique properties to be obtained.
However, nanomaterials are difficult to obtain for various reasons. The resulting nanomaterials are usually thermodynamically non-equilibrium systems with a defective structure, and excess surface energy causes the particles to stick together and aggregate.
In addition, nanoparticles are chemically active and often lose their unique properties when they interact with other substances [116].
The second way of producing composite materials based on fluoroplastic ensures that the components are mixed better.
Mixing takes place in solutions of alcohol, acetone, distilled water etc., sometimes surface-active additives are injected into the suspension to improve the interpartial interaction [117]. Uniform distribution of the filler in the matrix, which improves the physical and mechanical properties.
Composite materials are obtained in colloid mill mixers, which are widely used in domestic practice [118].
The static cold pressing of composite mixtures with a pressure of 50-70 MPa ensures their primary monolithization and gives the products a certain shape.
The final formation of the structure and properties of composite materials takes place during sintering at temperatures of 360-390 ° C and a holding time of 15-20 minutes per mm sample thickness.
Sintering can either be free or under pressure. The pressure is generated by external stress or by limiting the thermal expansion of the molded part during sintering in a closed mold, as a result of which a greater interaction of the components of composite materials and a lower porosity of the material is achieved. An important factor is the cooling rate of the workpiece after sintering.
At low cooling rates, a denser structure is formed, which ensures increased strength properties.
1.4.2 Manufacturing techniques for metal-filled composites Polyethylene matrix materials
The choice of polyethylene as the polymer matrix is associated with high strength, crack resistance in aggressive environments, heat resistance, frost resistance, low specific weight, the ability to transmit ultraviolet rays and absorb radioactive radiation, good dielectric properties, good recyclability in products.
Due to this combination of properties, low-pressure polyethylene is widely used in the chemical, petroleum, electrical engineering, coal, aviation, forestry, wood, light and food industries, heavy and traffic engineering, medicine, agriculture, machine tools, instruments and Shipbuilding etc.
The use of low-pressure polyethylene as molds for metal-polymer composite anti-friction materials is not only due to economy, but also contributes to technical progress - reducing the weight of products, increasing their service life, reducing the labor intensity of production, etc. [119- 122].
The most important methods for processing composite materials with a low-pressure polyethylene matrix are injection molding and extrusion.
The cheapest method of manufacturing thermoplastic polymer products is injection molding.
Despite the fact that the cost of the equipment in this process is high enough, its undeniable advantage is its high productivity.
In this process, the metered amount of the melted thermoplastic polymer is injected under pressure into a relatively cold mold, where it cures as the final product. The process consists of placing a compounded plastic material in the form of granules, pellets or powder from a bunker at certain intervals in a heated horizontal cylinder, where it softens. The hydraulic piston provides the pressure required to push the molten material through the cylinder into a mold at the end of the cylinder.
As the polymer mass moves along the hot zone of the cylinder, liquid phase mixing is performed.
Composite material with a worm screw, which ensures that the filler is distributed evenly over the entire volume of the matrix.
The molten plastic material is then injected through a mold hole into the mold base.
In its simplest form, the form is a system of two parts: one part is in motion, the other part is stationary.
The stationary part of the mold is attached to the end of the cylinder and the movable part is removed and put on.
A special mechanical device is used to close the mold tightly. At this point, molten plastic material is injected at a pressure of 1500 kg / cm2. The mechanical closure must be carried out in the same way to withstand high operating pressures. Uniform flow of molten material in the interior of the molds are preheated to a certain temperature. This temperature is usually slightly below the softening temperature of the plastic to be pressed.
After filling the mold with molten polymer, it is cooled with circulating cold water and then opened to remove the finished product. This cycle can be repeated many times in both manual and automatic mode [123-125].
The supramolecular structure of polymeric products mainly determines their physical-mechanical and operational properties, which can be controlled by introducing various modifying substances into the material [126-128].
The authors of the works [129] examined the influence and technological parameters of modifying additives on the structure and properties of low-pressure polyethylene as a matrix for composite materials obtained by extrusion. It is known that those responsible for the properties of extrusion products are the surfaces that cause their structural heterogeneity and concentrate the total stresses.
The composite material from the initial low-pressure polyethylene is characterized by an inhomogeneous, supramolecular structure typical of extrusion products [130].
At a screw speed of 20 rpm, the composite material of the outer surface is characterized by a deformed structure, the inside with round, well-defined spherulites. The deformation of the structural components decreases with increasing speed.
Undeformed spherulites with a diameter of 3-4 micrometers are observed on the inner surface of the samples under consideration.
Consequently, a method of extrusion through the increased physico-mechanical indicators technological process at minimum screw speeds should be issued for the inclusion of the composite materials through a polyethylene matrix, which significantly reduces the screw performance.
Chapter 2 Materials, equipment and methods of experimental research
2.1 Plant for the production of ultra-dispersive copper powder
In order to obtain ultra-dispersive copper powder, a system was developed, the overall view of which is shown in Figure 2.1.
Figure 2.1 - General view of the plant for ultradisperse copper powder
The principle of operation is based on the electrochemical method for obtaining powder, which, due to the variation of the electrolysis conditions, the current density and the electrode potential, enables the control of the speed of the electrode reactions and, due to this productivity, the chemical composition and additional additives (stabilizers, complexing agents, etc.) Size and shape of the powder produced.
The universality of the method is that it enables the use of compact metals, alloys, oxides, salts, including metal-containing materials, which can be recycled in the form of scrap, as a raw material base.
Process diagram of the plant for the production of ultra-disperse copper powders
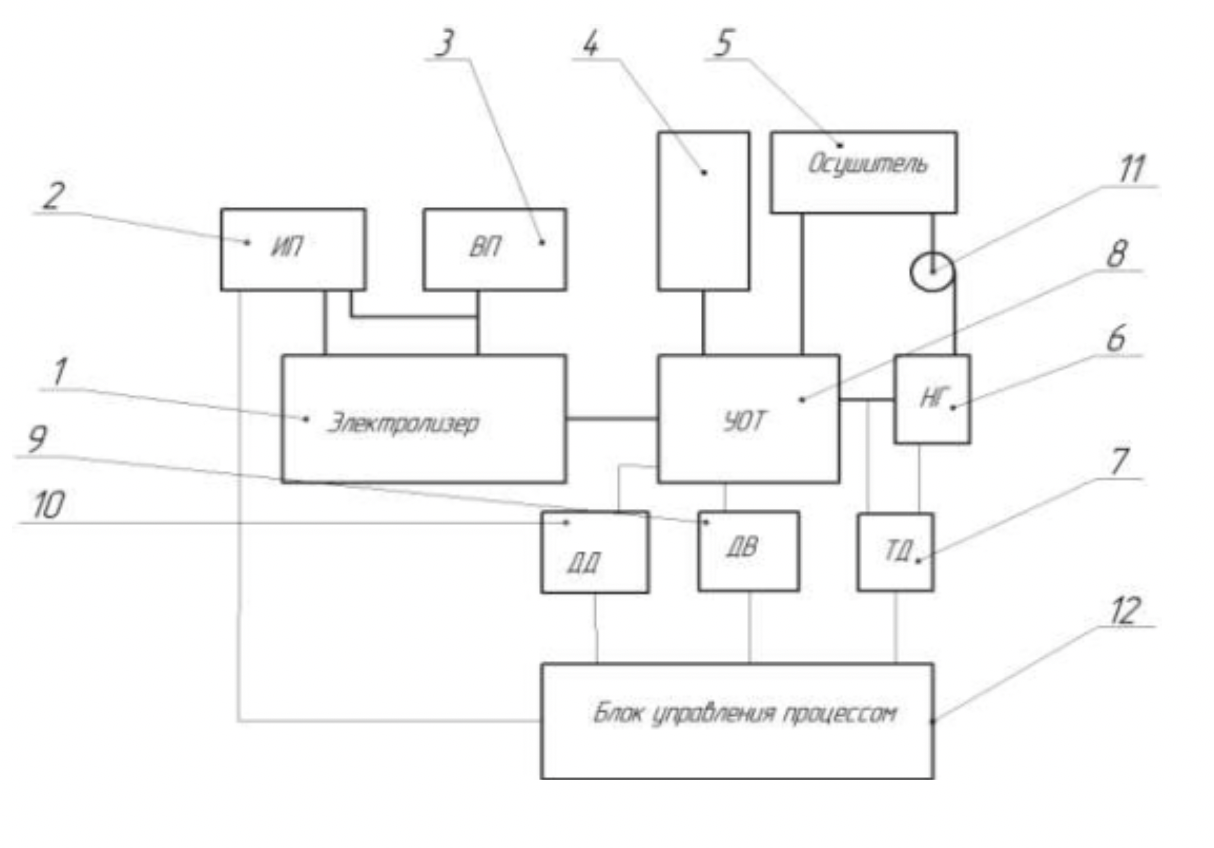
The technological scheme of the device for the production of ultra-disperse copper powders can be represented in the form of three blocks: an electrolysis block, a block for the separation of the electrolyte and the drying of ultra-disperse powders, the process control unit.
Figure 2.2 - Diagram of the core installation for the production of stabilized ultradisperse copper powders
1 - electrolyzer; 2 - power source; 3 - vibratory drive; 4 - distillate tank; 5 - moisture adsorbers;
6 - gas heating;
7 - thermoregulator;
8 - separator;
9 - humidity sensor (hygrometer);
10 - pressure sensor;
11 - compressor; 1
12 - process control unit
Electrolysis unit, consisting of an electrolysis cell with a corrugated cathode, which is equipped with a vibration drive, an anode and a power source.
Ultra-disperse device.
Electrolyte powder, washing and drying, contains a device for separating ultradisperse powder suspension, a container with distilled water and a closed drying system with a gas heater, a compressor and an adsorber to remove moisture from the circulating gas.
The control unit for processes for the extraction of ultra-disperse copper powders is executed via the control unit to which it is connected:
Sensor to control the gas temperature,
Ultra-disperse powder moisture sensor, pressure sensor and power supply control module.
Electrolyser for powder production
Copper powder is obtained by electrolysis on a titanium riflenoma vibrocatode with soluble copper anode in pulse mode with a pulse amplitude of 0,2 A / sm2, pulse duration and pause 1: 1 s.
The electrolyte change is carried out periodically every 2 hours of the electrolysis.
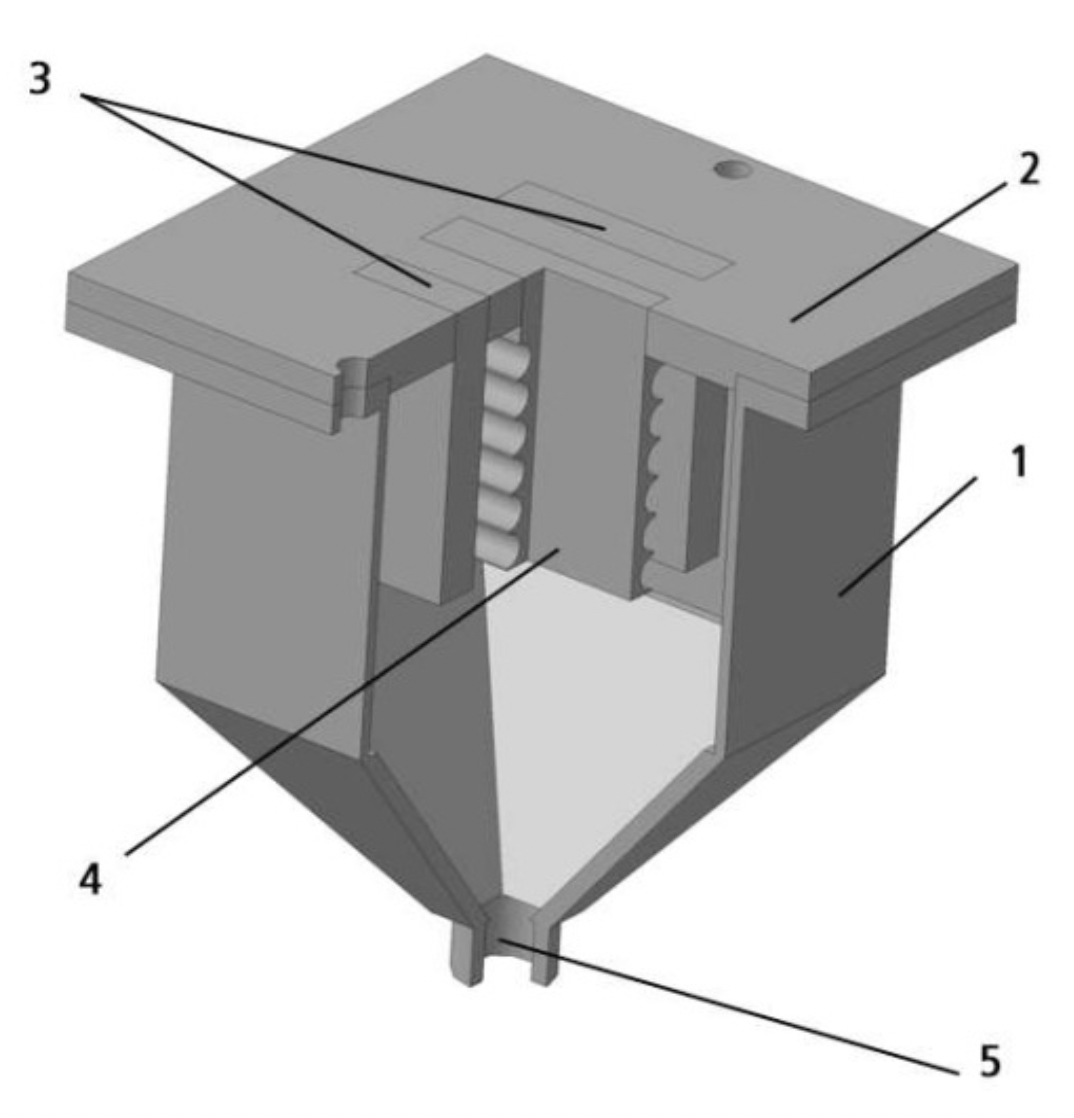
The electrolyzer (Figure 2.3) has a prismatic shape with a pulse assembly that forms a pyramid in the bottom.
In the prismatic part of the electrolyzer there are corrugated cathodes and graphite anodes, this part is equipped with electrolyte swing connections. The pulse assembly is connected to the powder separation device via a ball valve.
Figure 2.3 - Electrolysis for powder production
1 - electrolyzer body;
2 - lid;
3 - anodes;
4 - cathode;
5 - drain fitting.
The electrolysis was carried out on vibrating cathodes with different concentrations of stabilizing additives in the electrolyte.
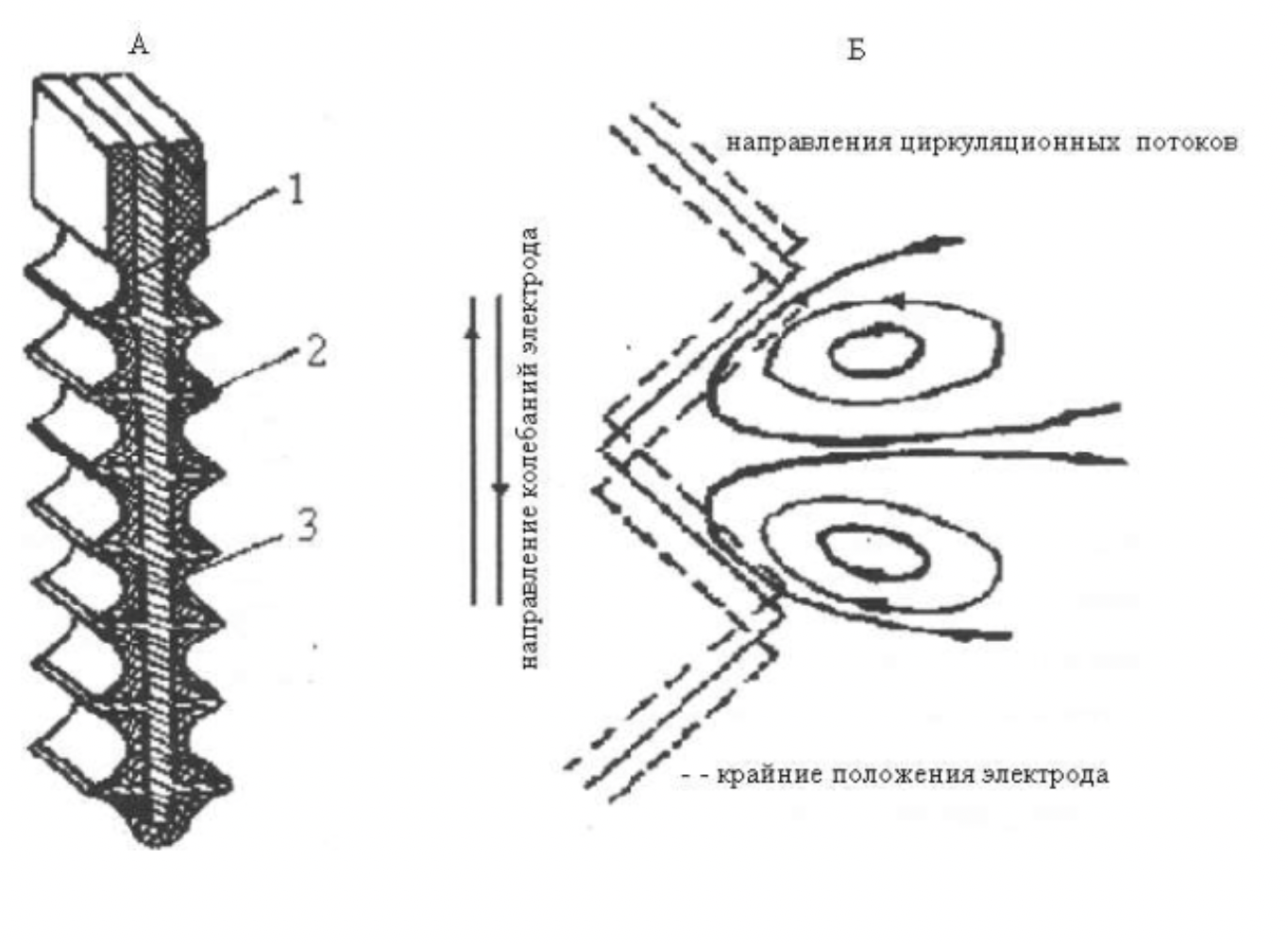
Figure 2.4 - Corrugated cathode with partially isolated corrugations
1 - cathode plate,
2 - cathode work surface,
3 - insulation
- and the circulation flows resulting from their fluctuations (B)
The choice of the cathode shape is justified by the earlier studies presented in [131].
The cathode vibration promotes the generation of turbulent flows in the electrode layer, whereby the powder particles are separated from the cathode surface and crumble to the bottom of the electrolyzer. To form ammonia complexes from copper, ammonium chloride is introduced into the electrolyte composition, the copper anode is dissolved to form ammonia complexes from copper (I), which are restored on the vibrocathode to form nanoparticles from powder.
It is ammonium chloride at the expense of its buffering properties to prevent alkalization of the electrolyte layer.
The choice of the cathode current density depends on the achievement of the maximum performance on copper powder.
At high current densities, a chemical reaction reduces the productivity of copper oxide (I) formation as a result of the leaching of the layer close to the cathode.
The anode current density is chosen so that salt passivation of the copper anodes is excluded.
Molecules made of polyvinylpyrrolidone or polyacrylamide, which are injected with the electrolyte, are sorbed onto the powder surface and form a protective film that slows down their growth and agglomeration.
Device for separating and drying powder
Metal powder is separated by filtering argon inert gas under excess pressure on the filter partition.
After the powder has been separated off, a washing process is carried out in order to finally remove the traces of electrolyte from the surface of the powder part.
The rinsing is carried out when distilled water is added to the filter,
The end of the washing process is determined by measuring the electrical conductivity of the washing water; after washing, the metal powder is dried by blowing with hot inert gas heated to a temperature of 90-1100C.
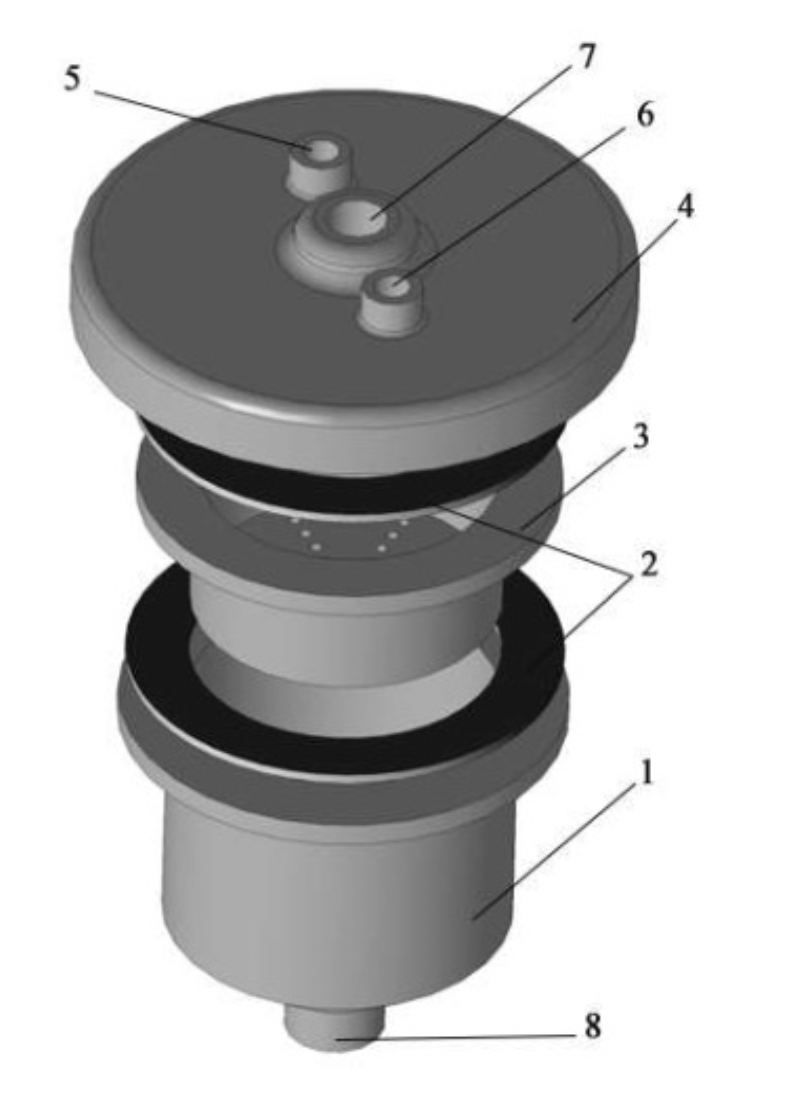
Figure 2.5 - Powder separation device
The suspension of metal powders is separated by a device consisting of (Figure 2.5) a container 1 with a removable filter device
Partition 3, removable cover 4, which is mounted on the filter via O-rings 2, equipped with an inlet nozzle 7 for supplying the sludge
Powder on the filter screen, connection 5 for the detergent supply
Water and inert gas connection 6, the bottom of the housing is in a conical shape, equipped with a drain 8 for removing liquid from circulating gas.
The device for separating suspensions of ultradisperse powder will be in contact with aggressive media, the housing (Figure 2.5) of the device is made of rolled stainless steel of quality class 03H16N15M3, GOST 5632-72.
The removable filter screen 3 and the cover 4 (Figure 2.5) are made of steel grade 40X GOST 4543-71 with a corrosion-resistant nickel-phosphor coating, which protects the surface from destruction.
Sealing rings are made of heat-resistant silicone rubber that works at temperatures from -100 to +300оС can be operated. A hygrometer for measuring air humidity and temperature was used to implement the proposed method for controlling the drying kinetics.
The proposed method for using the hygrometer to control the drying of the powder is as follows: during the drying process, a gas heated to a certain temperature is passed through the powder, and after a while the sorption equilibrium between the liquid on the powder surface and the gas flowing through is established in volume. Thus the moisture of the gas will be proportional to the moisture of the material contained. The basis for the use of the sorption equilibrium in a closed room for the purpose of material moisture control was the so-called volume method of heat and mass exchange known in theory and practice. Circulating gas after removal
from the dehumidification chamber goes into filter adsorber to remove moisture from it.
Anhydrous potassium chloride was chosen as the adsorbent for moisture absorption from circulating gas, due to its properties for moisture adsorption, produced in the form of a porous membrane. After drying, the gas returns to the heater, where it is heated to a temperature of 90-110oC and re-enters the powder dryer.
Managing the process of extracting ultradisperse powders and minimizing the human factor on the process of extracting, automated controls have been used that allow you to control the process yourself with minimal human involvement.
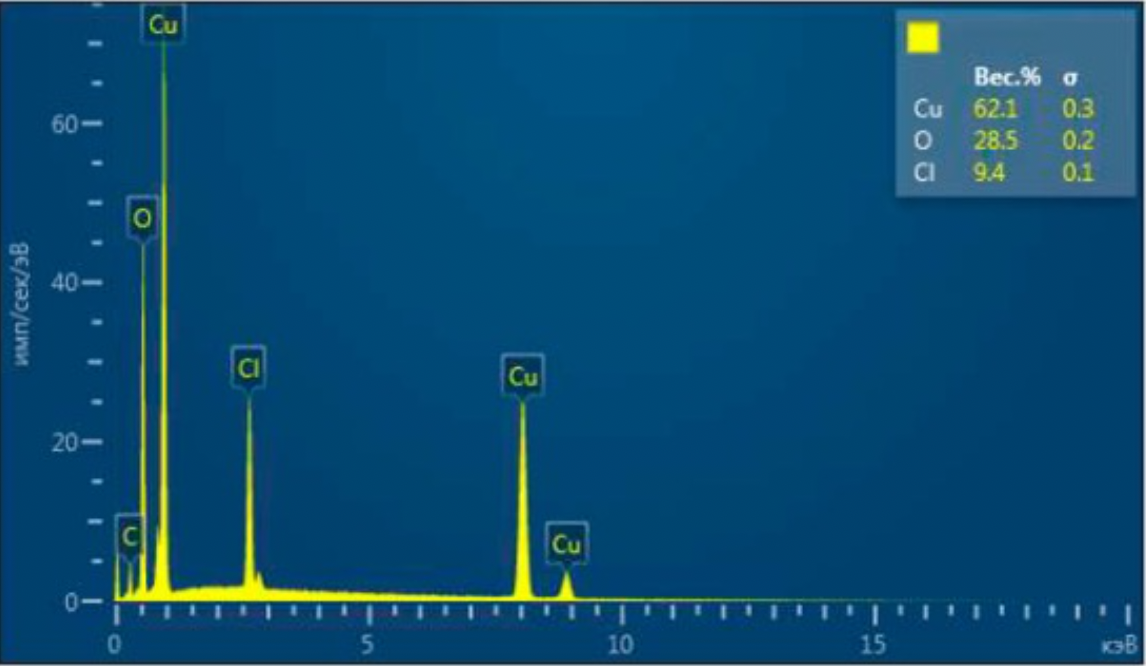
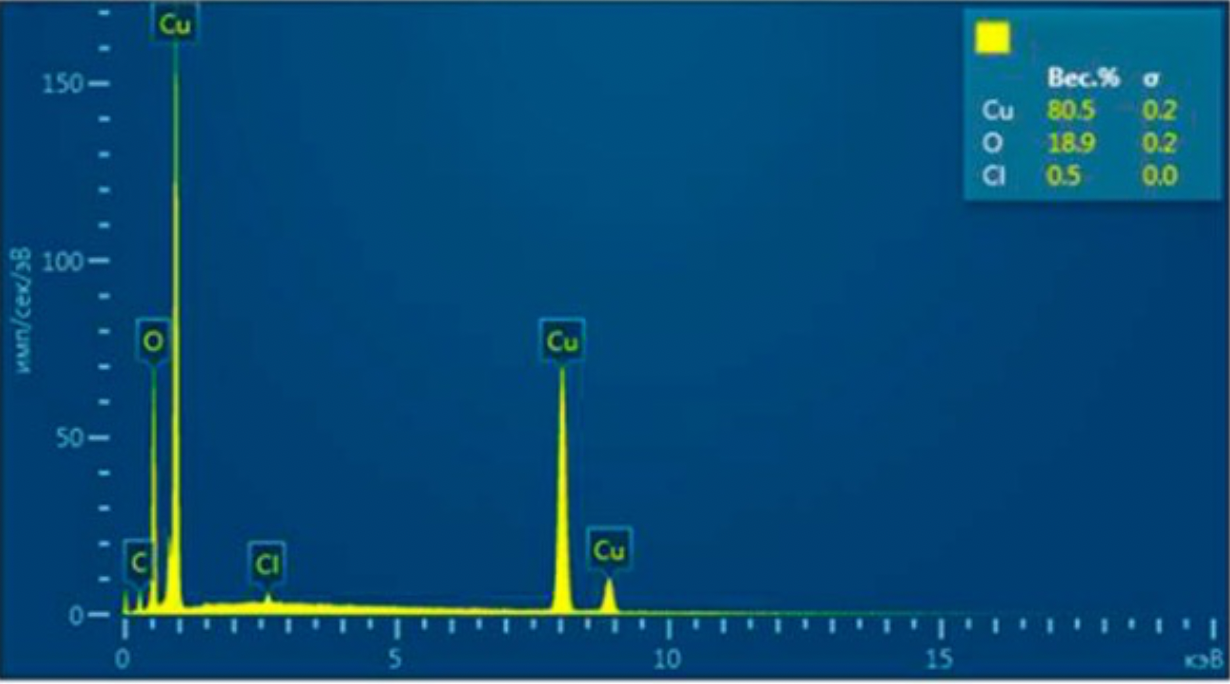
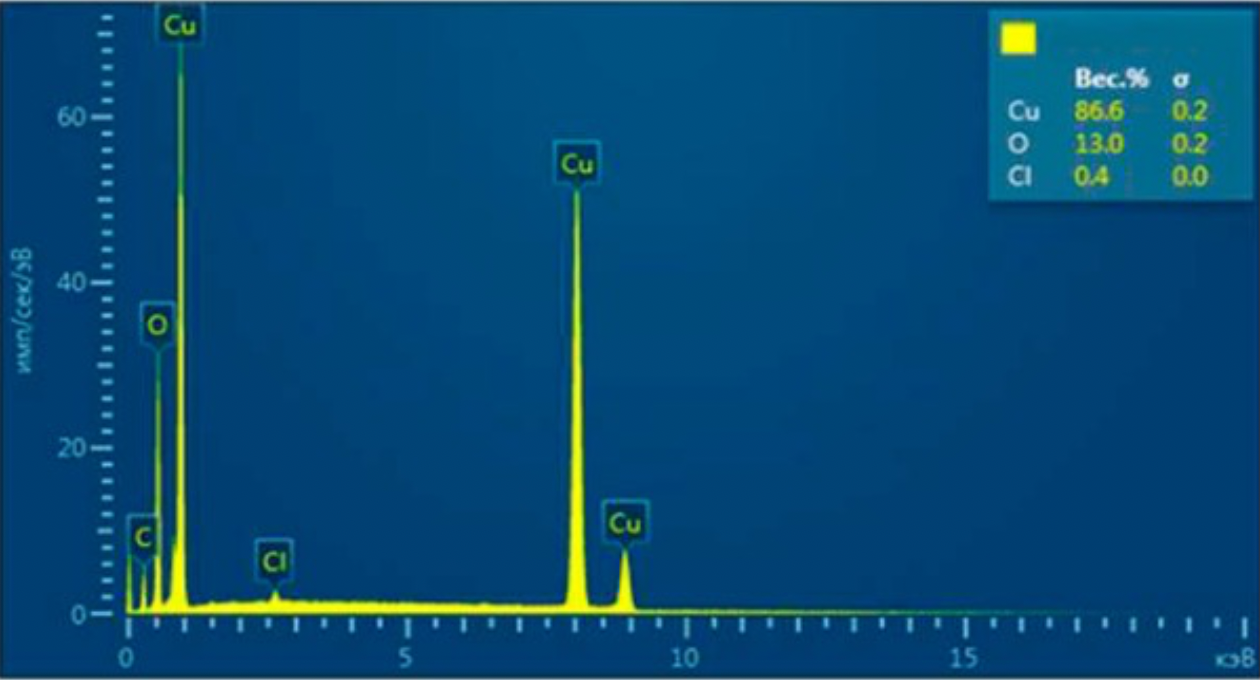
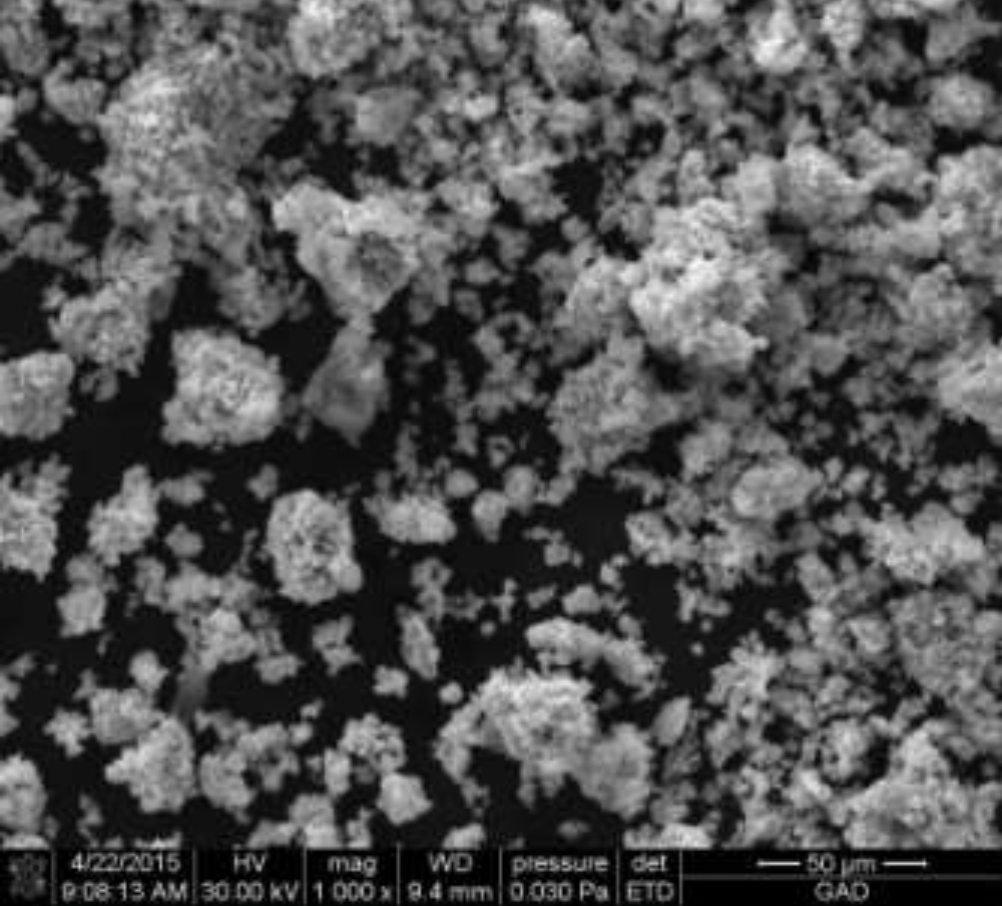
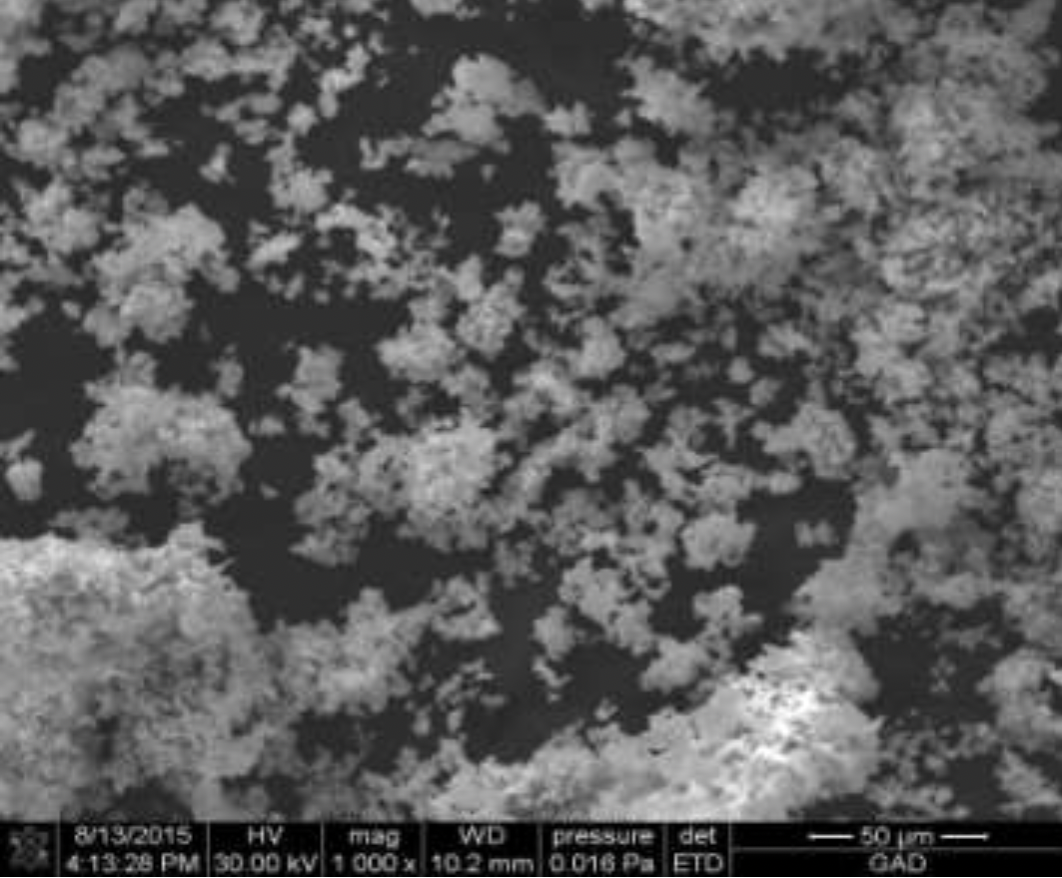
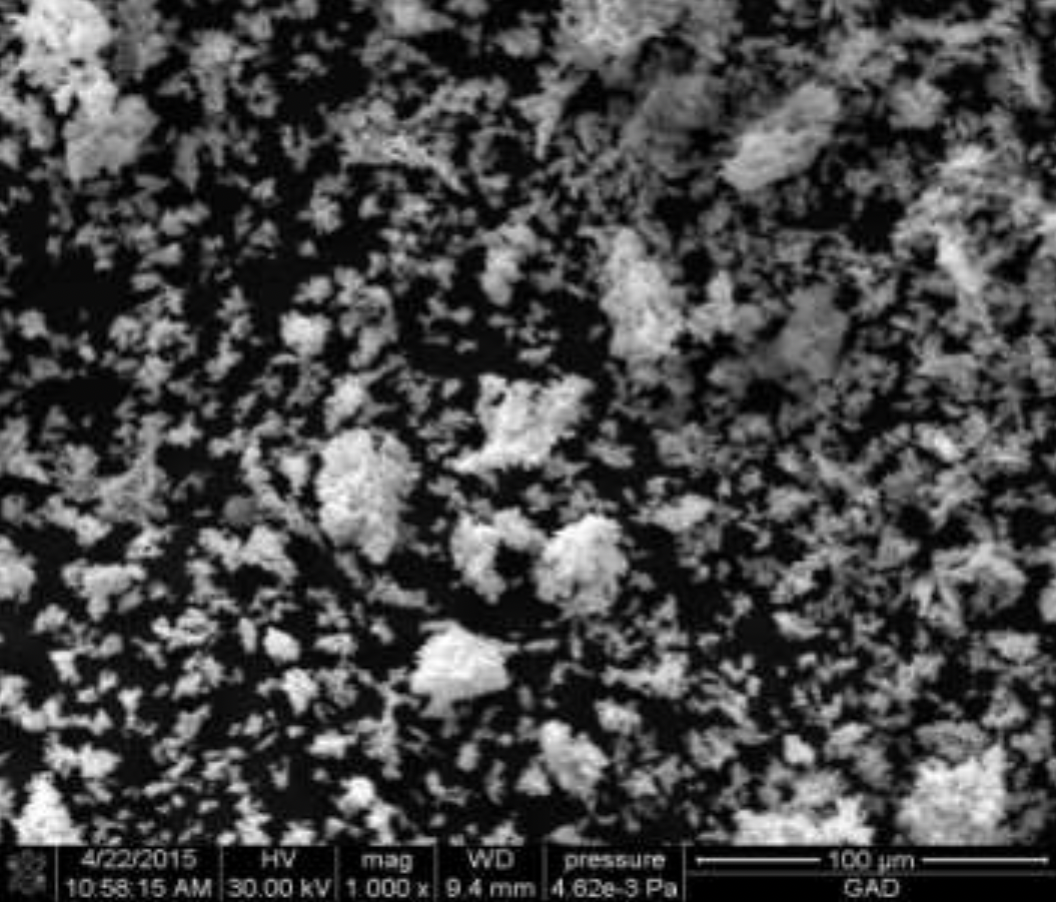
2.2 Methods for examining the composition and properties of the ultradisperse copper powder obtained
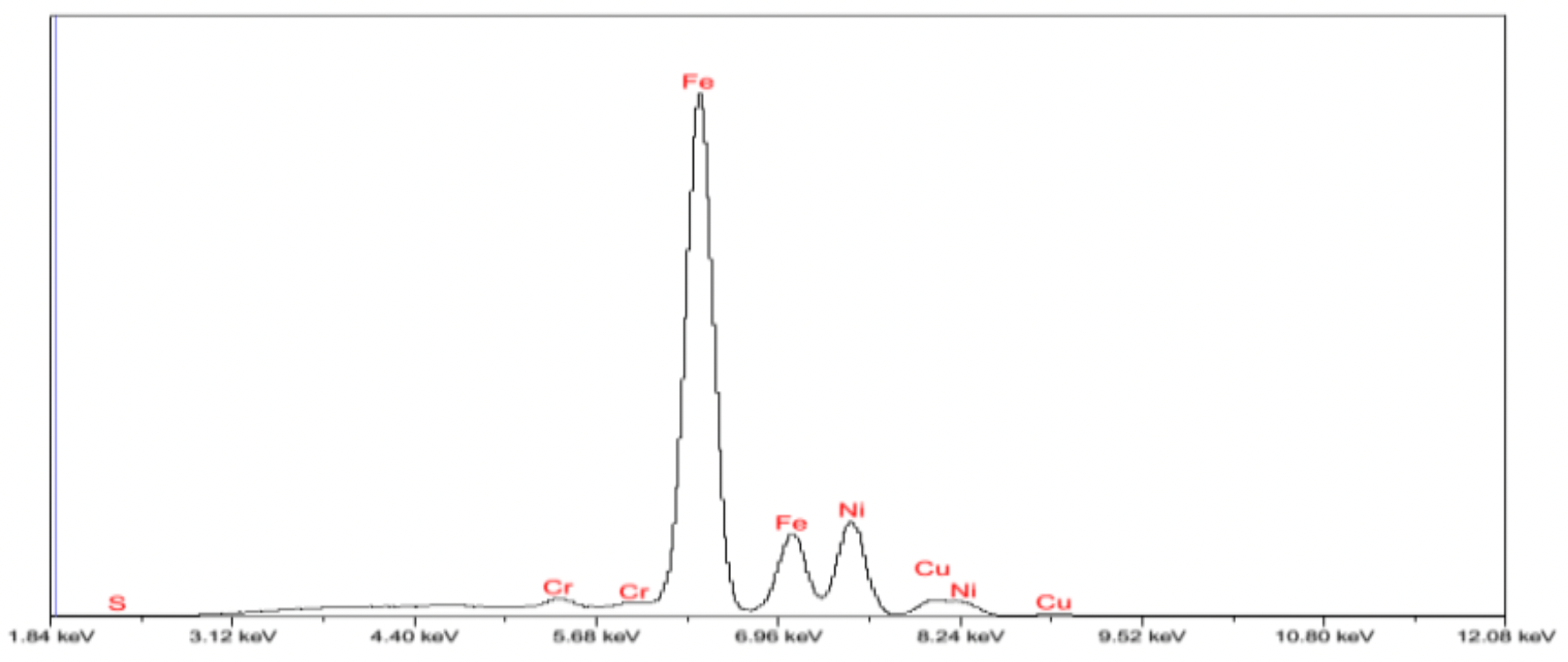
The structures obtained by ultradisperse powder were examined on the energy dispersive micro-analyzer EDAX GENESIS, which is compatible with computers and special software, the maximum magnification of the device was up to 150000 times, the resolution was 1 µm, working (accelerating).
Voltage - 30kV, and the detectable signal is secondary electrons. Reference samples were used for the analysis.
X-rays of copper, iron, carbon and other elements were excited with the focused electron beam at the examined point of the material. The volume of the material examined was determined by the radiation intensity.
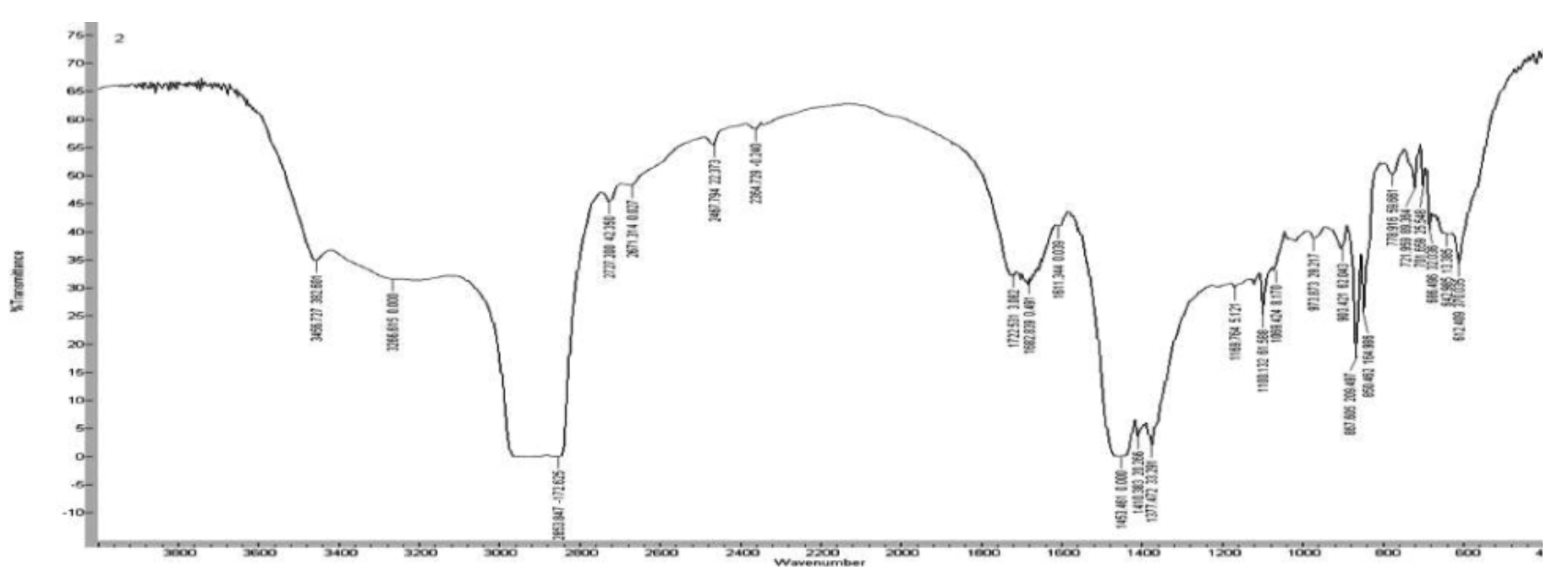
IR spectroscopy methods
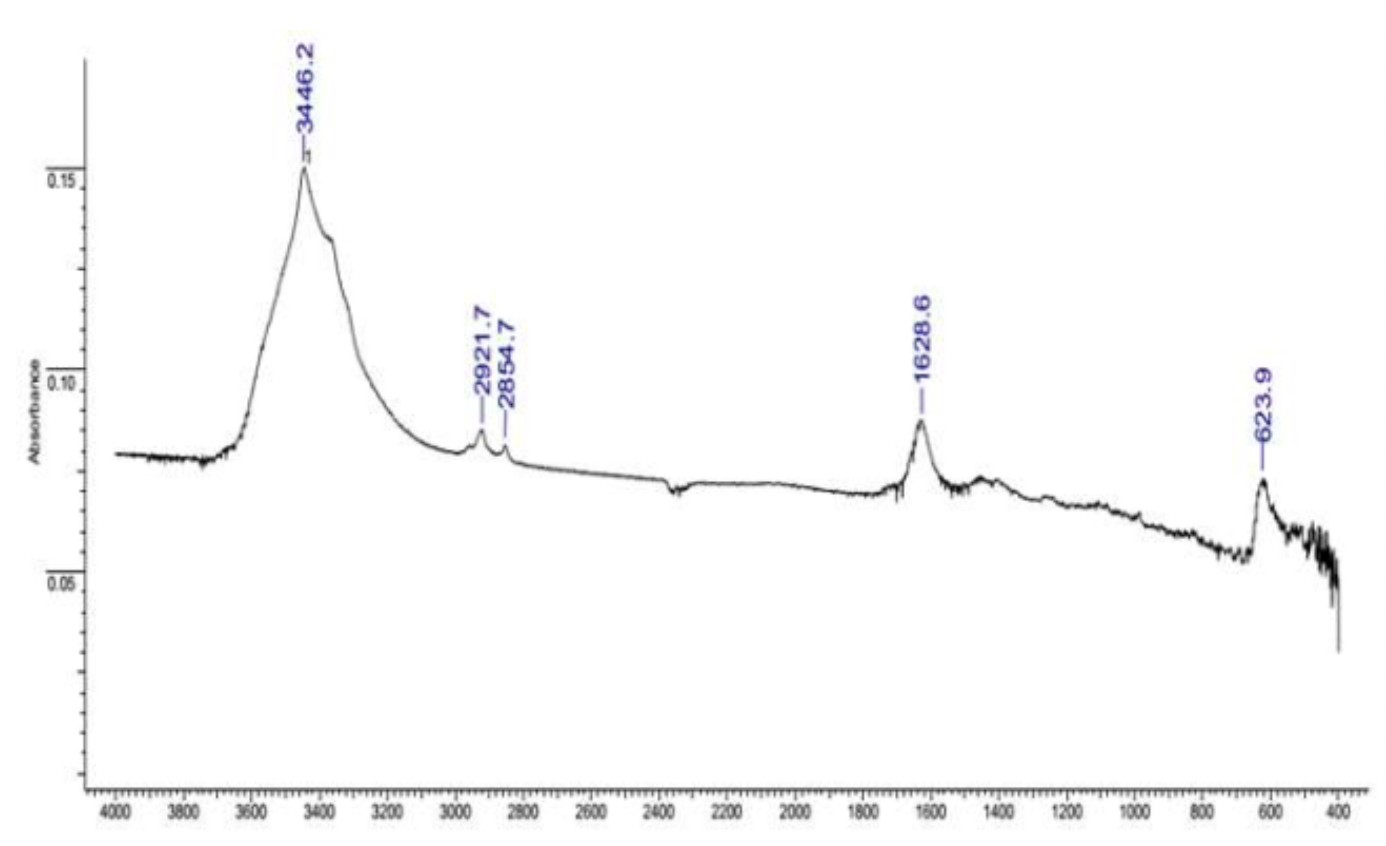
Coordination compounds formed in the course of the extraction of ultradisperse powders with water-soluble polymers were investigated, the investigation was carried out on the Varian 640 device.
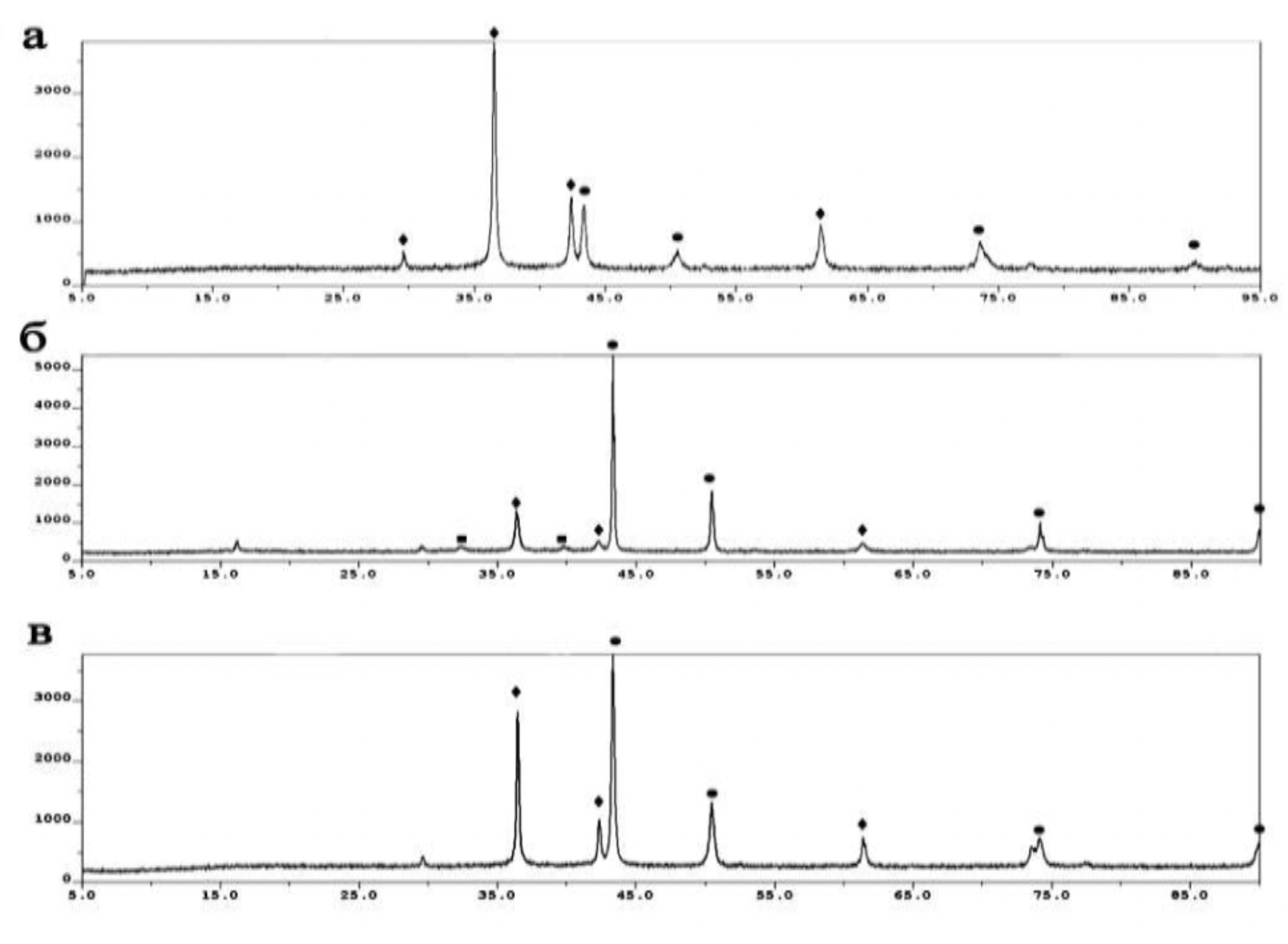
The X-ray phase analysis of powders was carried out on the X-ray diffuser.
ARL X'TRA Thermo Fisher Scientific diffractometer using the method described in [132]. The diffractograms were recorded at a rate of 1-2 degrees per minute.
Their decoding and phase identification were carried out according to the established method [133, 134] using the corresponding reference data [135].
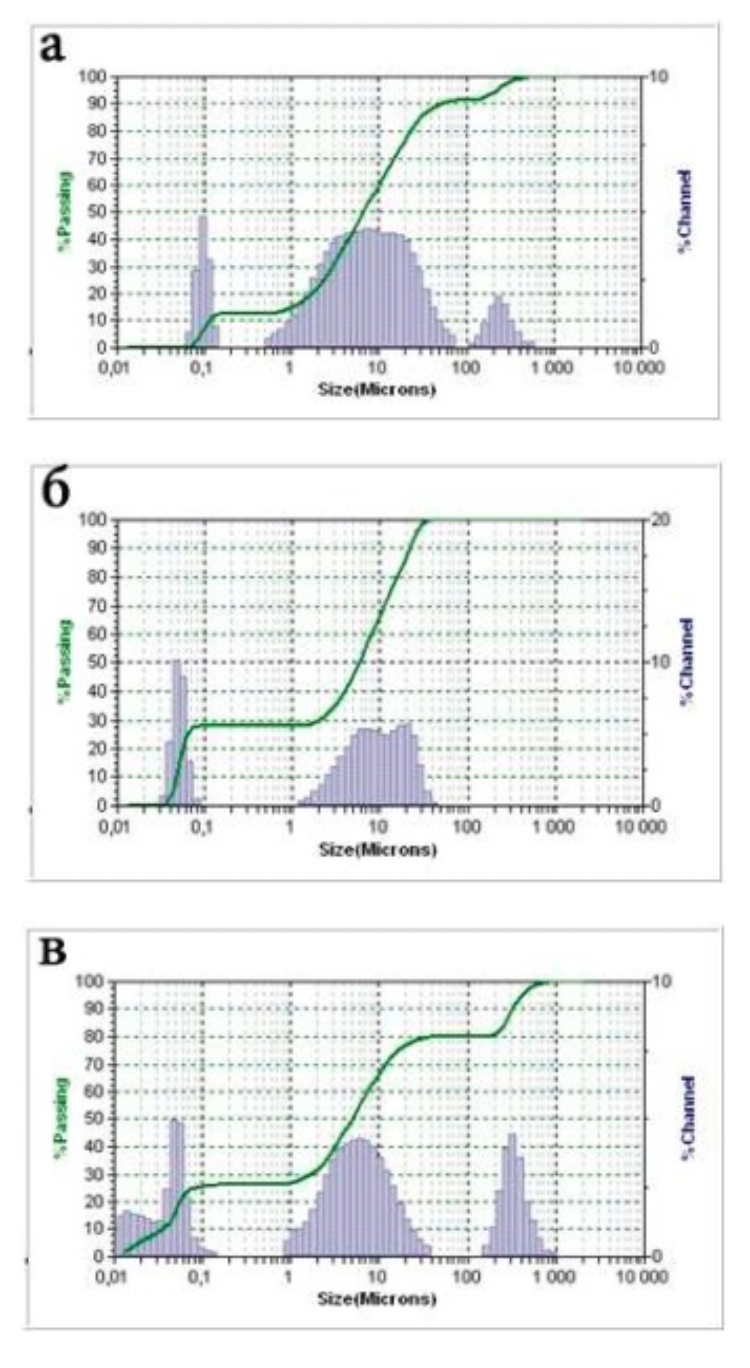
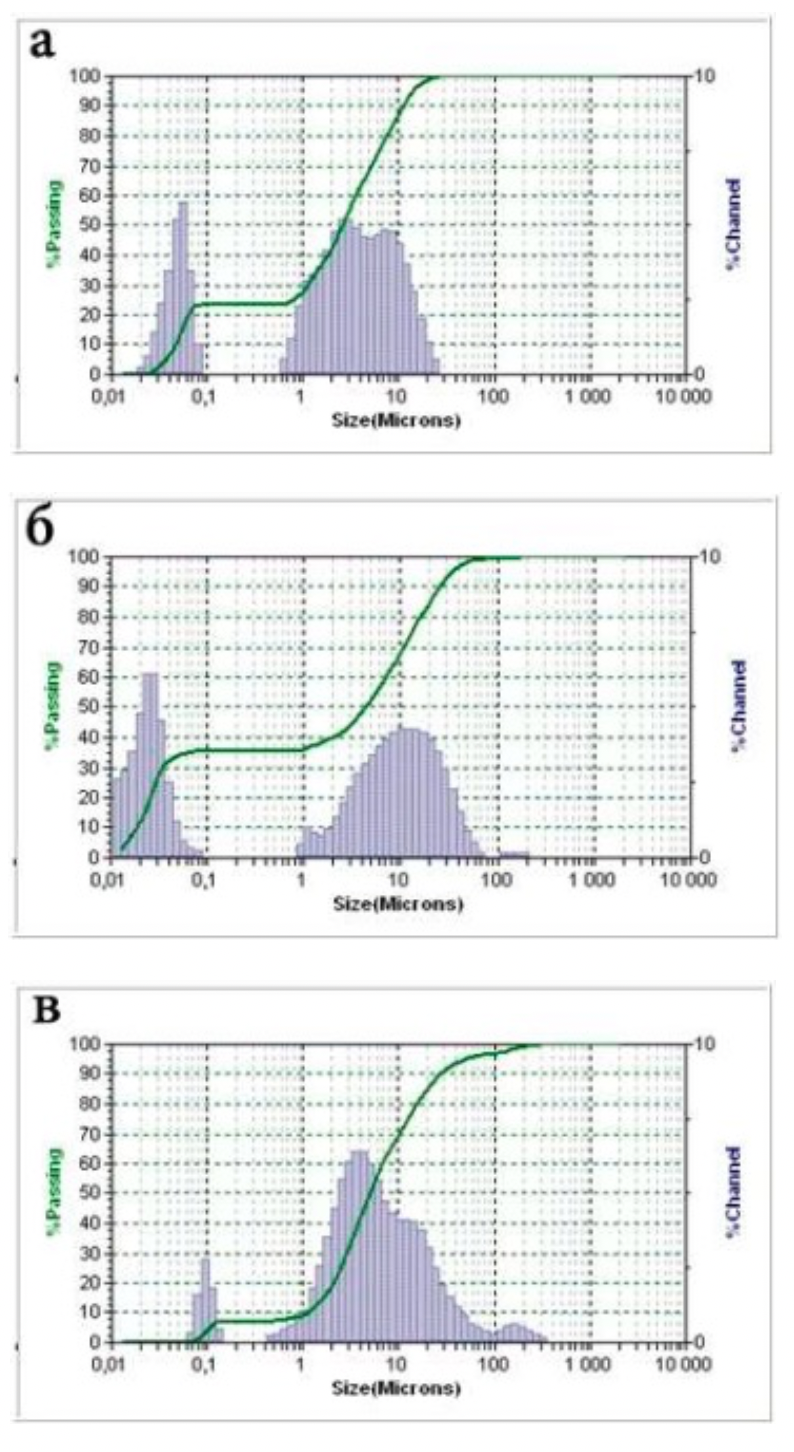
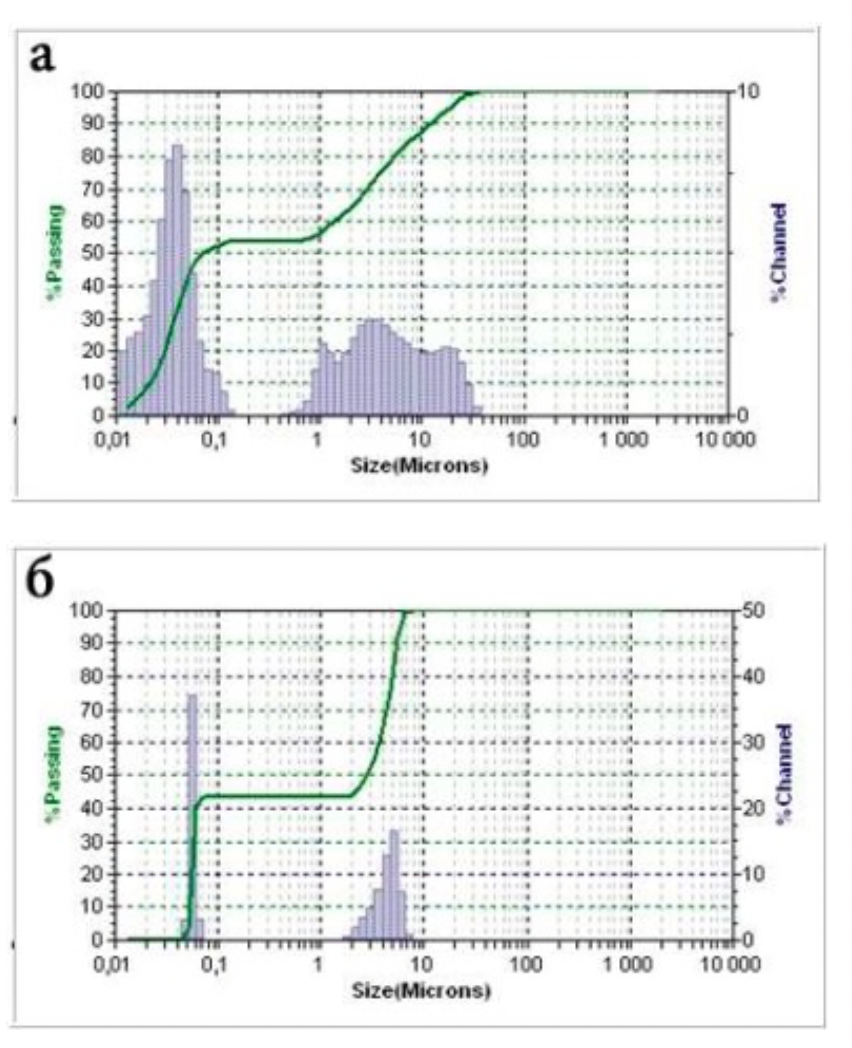
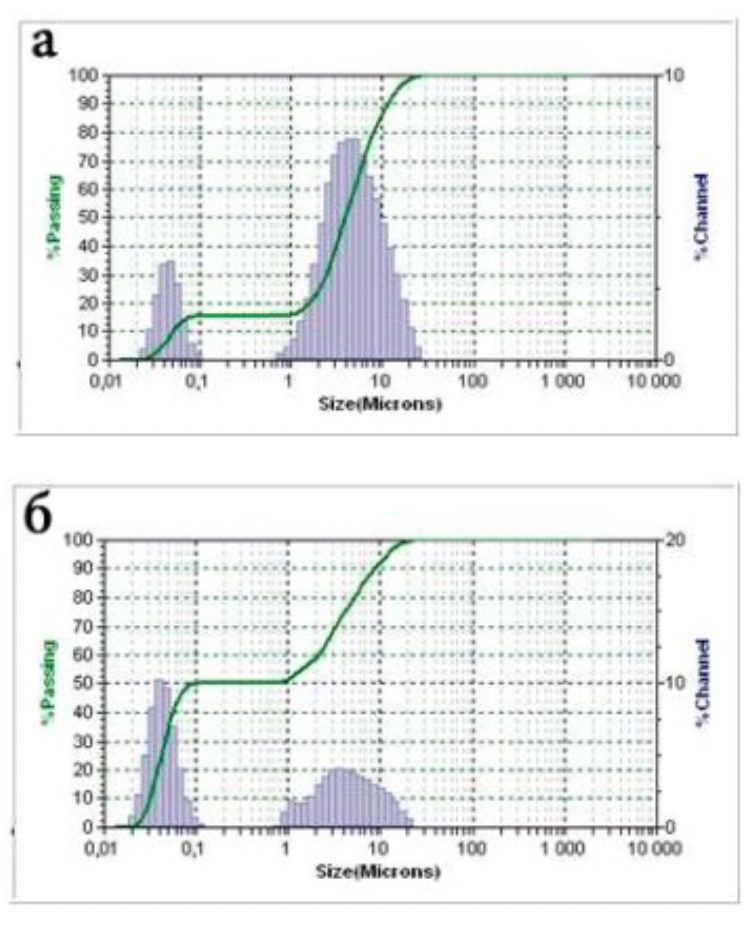
The standards of the diffraction spectra were selected from the form file which was accepted by the American JCPDS Powder Diffraction Data Standardization Committee and has a measurement error of plus / minus 5%. The particle size distribution was determined using the Microtrac S3500 device, equipped with a laser analyzer, diffractometer with technical properties (Table 2.1). Laser granulometer provides a reliable representation of particle shape and size through laser diffraction.
The patented three-laser system offers high accuracy, a large measuring range and the repeatability of the analysis results. There are no differences between repeated submicron analyzes.
The stability and self-adjustment of the system were based on an increased number of lasers, the latest result processing technology and a wide range of applications using modern light-sensitive detectors.
Laser analysis system (laser diffractometer) Microtrac S3500 meets the international standards for laser particle size measurement and is certified according to ISO 13320-1.
The device is registered in the State Register of the Russian Federation and certified according to ISO 13320-1.
Measuring instruments register (US.E.27.001.A 23120).
Table 2.1 - Technical data of the Microtrac S3500 laser analyzer and diffractometer
| precision
|
relative deviation (CV)
|
|
| Glass spheres 642 μm - 0,7%.
|
||
| Glass spheres 56 μm - 1,0%
|
||
| Glass spheres 0,4 μm - 0,6 | ||
| Standard analysis time | 10 - 30 seconds | |
| Measuring range | 0,021 - 2816 μm | |
| optics | Three solid-state lasers with a wavelength of 780 nm | |
| measuring angle | The measuring angle of 0,02-163 degrees is provided by a 151-element detection matrix. | |
2.3 Methods for the production of composite materials
To estimate the influence of the investigated powders on tribotechnical and physical-mechanical properties of composite materials,
Samples were made with the batch containing polymer (fluoroplastic-4 10-70% polyethylene-277 40-60%) and the powders prepared.
The fabric was mixed on the drum mixer and then pressed into cylindrical molds measuring 60 × 80 and 90 × 10 mm with a pressure of 150-25 MPa.
The static cold pressing was carried out on a hydraulic laboratory press in cylindrical forms with the dimensions 90 × 10 and 15 × 25 mm according to the scheme of one-sided pressing (Figure 2.6).
On the left side of the scheme, the position of the tool is shown before casting, on the right - after the load is applied.
Figure 2.6 - Form scheme for static cold press specimens
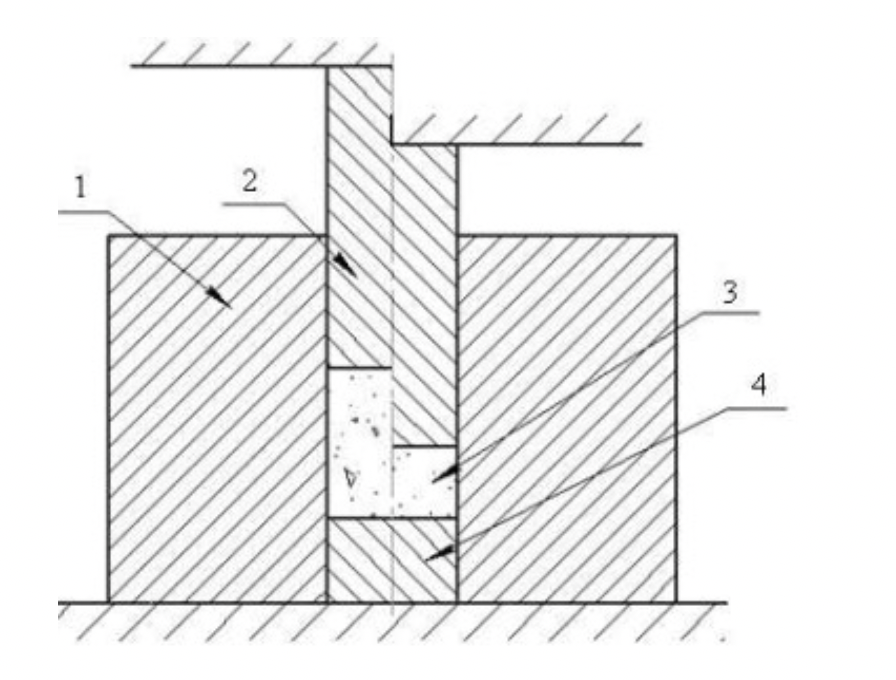
1 - die;
2 - upper punch;
3 - powder blank;
4 - lower stamp.
Sintering of samples intended to investigate mechanical properties and wear resistance was carried out in a chamber furnace at 390-400оС in an inert gas (argon) environment (Fig.2.7).
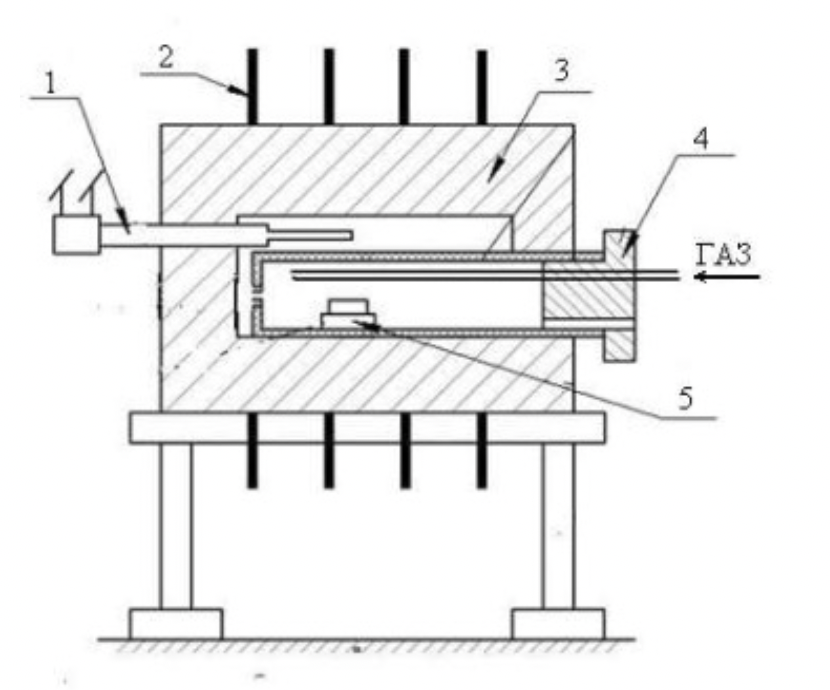
Figure 2.7 - Scheme of the electric laboratory furnace
1 - thermocouple;
2 - heating element;
3 - lining;
4 - container;
5 - sample boat.
The samples were placed in a boat 5 made of heat-resistant steel.
Boat 5 was placed in container 4, which was fed with inert gas, which was dried by a solid adsorber.
For this purpose, a mixture of polyethylene and copper powder was passed through the screw extruder, which increased the uniformity of the filler distribution in the entire polymer volume.
The resulting mixture was added to 125-140оС heated mold cast and molded at a pressure of 40-45 MPa. The temperature in the oven was measured with a thermocouple and a thermoregulator with an accuracy of plus / minus 10 oC monitors and regulates.
After sintering, the physical and mechanical properties of the composite matallo polymer materials obtained with fluoroplastic matrix were measured.
In contrast to fluoroplastic, polyethylene has a low viscosity at the melting point, which enables a liquid phase mixture of the composite material during sample preparation.
For this purpose, the mixture of polyethylene and copper powder was passed through a screw extruder, which made it possible to increase the uniformity of the filler distribution over the entire polymer volume. The resulting mixture was added to 125-140оС heated mold cast and molded at a pressure of 40-45 MPa.
When all conditions are met, the polymer will evenly coat the filler without leaving a pore in the final product.
The samples obtained were tested to determine the physical and mechanical properties.
2.4 Methods to study the structure and properties of sintered composites
2.4.1 Investigation of the structure of composite materials
The influence of the introduction of powders as an alloy component in composite materials based on fluoroplastic-4 and polyethylene-277 on the formation of the structure and surface topography of powders was demonstrated with the aid of the optical metallographic microscope MIM-8, equipped with an electronic eyepiece. To examine the surface layers, the samples were cut perpendicular to the wear surface and examined with the MBS-1 microscope.
The interaction of the filler with the matrix was examined with the Quanta 200 scanning electron microscope in the “Nanotechnology” * Central Processing Center.
* MI Platov South Russian State Polytechnic University (NPI)
Investigation of the structure of the surface layer of composite materials
was performed on a SolverHV scanning probe microscope.
2.4.2 Determination of the physical and mechanical properties of the material
The frictional properties of the materials obtained were examined on the TMT-25 final friction machine (Figure 2.8).
Figure 2.8 - TMT-25 friction machine
(a) The kinematic scheme of the final friction machine;
- b) friction unit.
1 - friction unit;
2 - loading mechanism;
3 - head moving mechanism;
4 - friction clutch;
5 - planetary gear;
6 - belt drive;
7 - belt tension regulator;
8 - electric motor;
9 - bed;
10 - load-bearing frame attachment;
11 - frame body;
12 - counter body holder;
13 - sample holder;
14 - centering ball;
15 - spindle;
16 - rotating mechanism;
17 - pattern;
18 - screw for fastening the counter body;
19 - counter body;
20 - bearing.
The tests were carried out at loads of 0,5 to 5 MPa with a load change interval of 0,5 MPa and a sliding speed of 0,075 m / s both without lubrication and in MS-20 oil.
A roller with a diameter of 40 mm and a thickness of 0,7 mm made of P6M5 steel (Interstate Standard 19265-73), which was hardened to 61-65 HRC, was a contretel.
The coefficient of friction was determined mathematically by the measured force (frictional torque), which was continuously recorded during the tests.
where f is the coefficient of friction;
N is the moment of friction;
P - specific load, MPa;
S - friction area, cm2;
Determination of wear and tear
The samples were tested for 10 minutes and the control test for 30 minutes.
Wear was determined by changing the linear dimensions and mass of the samples before and after 1, 2 and 4 hour tests.
The tests were carried out on the final friction machine TMT-25 with a relative sliding speed of 0,075 m / s. And the load was 4 MPa. The tests were carried out without lubricant in the friction zone.
The linear wear is determined by changing the linear dimensions with the help of an optical measuring device with an accuracy of 0,003 mm.
Weight wear was determined on an analytical balance with an accuracy of 0,001 g.
The weight wear is determined by the formula:
W = W1 - W2
где W1 - вес образца до испытаний, grams.
W2 - вес образца после испытаний, grams
The strength properties of the developed composites were determined on a P-0,5 breaking machine (state standard 25.602-80).
The hardness was determined on the AS-111 device in accordance with the state standard 4670-91.
2.5 Materials used
The influence of water-soluble polymers on the production of ultradisperse copper powder and the properties of metal-polymer composites was investigated in the work.
The thermoplastic polymer polyethylene 277 and the fluoroplastic-4 were used as the polymer matrix, with high chemical resistance and practical relevance for the extraction of promising composite materials based on them.
Table 2.2 - Materials used
| material | State standard * / GOST * / TU |
| PTFE 4 | GOST 14906-77 |
| Polyethylene 277 | GOST 16338-85 |
| polyacrylamide
|
TU / Technical Specifications 2216-042-07510508-2009 |
| Copper | GOST 859-2001 |
| ammonium chloride
|
GOST 3773-72 |
| Ammonia solution 25% | GOST 6221-90 |
polyvinylpyrrolidone
Table 2.3 - HDPP characteristics
| No. | The name of the indicator
|
The norm |
| 1 | Description
|
Powder white or white with light
yellowish hue with a weak specific smell. It is hygroscopic. |
| 2 | solubility
|
Easily soluble in water, alcohol,
Chloroform, in which ether is almost insoluble. |
| 3 | PH value | zwischen 3,0 und 7,0 |
| 4 | The transparency of the solution | 5 ml of solution A must withstand comparison with reference solution I. |
| 5 | Average molecular mass value
12600 2700 ± |
12600 2700 ± |
| 6 | Water,%, not more than | 5,0 |
| 7 | Alfapirrolidone,%, not more than | 3,0 |
| 8 | N-vinyl pirrolidone,%, not more than | 0,2 |
| 9 | Aldehyde,%, not more than | 0,2 |
| 10 | Sulfate ash,%, no more than | 0,05 |
| 11 | Heavy metals,%, no more than | 0,0005 |
| 12 | Hydrazine,%, no more than | 0,0005 |
| 13 | Peroxide compounds,%,
not more than |
0,04 |
| 14 | The toxicity | is not toxic. |
Chapter 3: Obtain stabilized ultra-disperse copper powder
3.1 Parameters for obtaining stabilized ultradisperse powder
An important task when creating composite materials with Ultradisperse powder as fillers is their even distribution over the entire volume of the polymer matrix. A major disadvantage of nanoscale powders is their high surface energy, which leads to agglomeration. It is practically impossible to distribute such powders in the matrix and mix them evenly using standard methods, so it is an important task to look for powder protection against agglomeration and aggregation.
Therefore, one of the most commonly used methods is the stabilization of ultra-disperse powders, the formation of a protective shell on the surface of the particles. To create a protective cover, water-soluble polymers were used in this work, which are capable of chemisorption on the surface of particles of ultradisperse powder during the recording.
The properties of such powders can differ significantly from unstabilized ultradisperse powders.
Ultradisperse copper powders, which are encapsulated in a protective jacket for stabilization and protection against agglomeration and aggregation, were obtained by electrolysis with a soluble anode.
By varying various process parameters, such as: concentration of electrolyte solutions, changing the current density, electrolysis time and drying temperature, it is possible to obtain copper powder with certain dimensions and properties.
As water-soluble polymers polyvinylpyrrolidone and polyacrylamide selected. Table 3.1 shows the optimal electrolyte compositions and parameters for the production of ultra-disperse copper powders.
These compositions were selected as optimal based on experimental data.
Table 3.1 - Compositions of the optimal electrolyte solutions and parameters for obtaining ultradisperse powders.
| No. | components
|
Content, g / l | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | ammonium chloride | 55 | 55 | 55 | 55 | 55 | 55 | 55 | 55 | |
| 2 | polyvinylpyrrolidone | 10 | 12,5 | 15 | - | - | - | - | ||
| 3 | polyacrylamide | - | - | - | 2 | 4 | 6 | 8 | ||
| 4 | Water | The rest of | ||||||||
| Parameters for the production of ultra-disperse copper powder | ||||||||||
| Electrolysis parameters | Significance | |||||||||
| Cathode current density, A / cm2 | 0,4-0,8 | |||||||||
| Anodic current density, А / cm2 | 0,05-0,07
|
|||||||||
| Duration of electrolysis, h. | 2-3 | |||||||||
Depending on the type of electrolyte, the cathodic recovery processes of the copper forms available in the electrolyte differ considerably. In the electrolyte (ammonium chloride) of CA, the direct restoration of the four-coordinate ammonia complex (3.1 - 3.3) (first maximum, Table Hp.1) as well as the restoration of copper oxide (I) (second maximum) (3.4) was observed, apparently from simple charged complexes formed by the restoration of doubly charged complexes due to the alkalization of the electrode layer according to the scheme:
[(3) 4] XNUMX+ + 2 = + 43 (first maximum) (3.1)
[(3) 4] XNUMX+ + = [(3)] +-
2 [3) 4] ²+ + 2- = 2 + 43 + 2 (3.3)
2 + 2 + 22 = 2 + 4- (second maximum) (3.4)
During the course of the electrolysis, the concentration of the double-charged complexes, which is estimated by the maximum current value, initially decreased to zero and then increased.
At the same time, the number of 2 practically constant.
This change in character of the electrode reactants can be associated with changing processes of passivation and subsequent activation of the copper anode.
In the HACCP electrolyte, the recovery of the unbound ion in the electrode layer by partial recovery of copper (II) and subsequent oxide deposition in an alkaline environment:
2+ + 2 = (first maximum) (3,5)
2+ + 2- = 2 + 2 (second maximum) (3,6)
After a short period of increasing currents in the maximum recovery process of the unbound copper ions (II), a decrease in their concentration and their stabilization was observed.
As has been found, the result of the anodic dissolution of copper in an ammonia environment is the formation of ammonia complexes.
Fixed recovery potential corresponding to the unbound copper ions in the complex, may be related to the earlier dissociation of the complex, enhanced in HDPE film on the cathode surface. The stabilization of the maximum currents over time was due to the adjustment of the cathode and anode current outputs.
At the same time, in contrast to the ammonium chloride electrolyte, the amount of oxide Cu 2O-layer gradually reduced. The polyvinylpyrrolidone surface film changed the pH balance of the electrode layer, thereby gradually reducing the possibility of copper oxide formation in this film.
Table 3.2 - Maximum potentials of the voltamperometric dependence and their respective electrode processes
|
Electrolytic
|
Maximum potential | reference data | reaction |
|
ammonium chloride
|
-0,244 | -0,232 | [(3)4]2+ + 2- = + 43 |
| -0,6 | -0,366 | 2 + 2- = 2 | |
|
Polyvinylpyrrolidone-ammonium chloride
|
0,1 | 0,158 | 2+ + - = + |
| -0,357 | -0,366 | 2 + 2- = 2 | |
|
Polyacrylamide ammonium chloride
|
0,333 | 0,338 | 2+ + 2- = |
| 0,093 | 0,158 | 2+ + - = + | |
| -0,306 | -0,232 | [(3)4] 2+ + 2- = + 43 |
The peculiarity of the ammonium chloride-polyacrylamide electrolyte is the lack of recovery of Cu2 O.
The first maximum is associated with the recovery of free, double-charged copper ions (II), the second with the recovery of copper (II) to copper (I) and the third with the direct recovery of complexes.
Consequently, the effect of the polyacrylamide surface film completely precluded the formation of copper oxide and promoted the intermediate dissociation of copper-ammonium complexes.
In contrast to polyvinylpyrrolidone film, the direct reduction of ammonia complex ions was not excluded. Both the concentration of the copper ions and the concentration of the complex ions increase mainly with time, which can be related to the excess of the anode current through the cathode.
It also prevents the complex ion from affecting the strength of the bond with the surface as well as the ability to preserve the solvate shell through crystalline seeds.
One can probably imagine the process of discharging complex copper ions that occur in the polymeric microfilm of surfactant on the surface of the cathode, the structure and properties of which represent the matrix for the formation of crystalline embryos and their agglomerates.
3.2 Properties of ultra-disperse copper powders depending on the technological conditions of their manufacture
3.2.1 Influence of the production parameters on the particle size composition
According to the data in methodology 2.1, the electrolysis can be carried out both in pulse mode and in DC mode.
When the powder is electrolyzed in the pulse mode, the particle size of the powder is reduced.
Histograms of the particle size distribution, which were obtained at different current densities of the electrolysis (Fig.3.1), attest to the fact that when the powder is received in the pulse mode, the fraction of fractions of particles with sizes down to 0,1 µm at a cathode current density of 0,6A / cm2 is much larger than in the case of electrolysis with direct current with the same parameters.
The process in pulse mode allows the growth of the particles to be limited, by the intermittent process of pulses and pauses with a frequency of 1 Hz it is possible to obtain a powder with a smaller size than with direct current. In the second case, the particle formation process is continuous, gradually growing, which explains the dendritic shape of the particles, which is characteristic of most electrolytic powders.
The authors of [127] have proven that embryos develop at different speeds in different crystallographic directions during dendritic crystallization. For example, the maximum crystallite growth of metals and alloys with a cubic lattice takes place in three mutually perpendicular directions that correspond to the octahedral axes.
As a result, branches are formed - dendrite axes of the 1st order, which deviate from the crystallization center at certain angles.
With the further development of crystallization from the axes of the 1st order at a certain angle to them, transverse branches begin to grow - the axes of the 2nd, and from them axes of the third order and so on. In the course of our work we have found that the size of the dendritic branches in the production of electrolytic powders depends on only one factor
The speed of the copper recovery reaction and at certain intervals its crystal sizes can be adjusted based on the cathodic current density. Obtaining ultradisperse powders in pulsed mode makes it possible to slow down the copper powder recovery reaction by several orders of magnitude. This is due to the fact that the resulting dendrites practically “freeze”, at this point on the surface of the particles due to the high surface energy, polymer chemosorbs and thus absorbs the active centers of the dendrite structure formation and passivates the particle before further growth. It is known that water-soluble polymers of polyvinylpyrrolidones and polyacrylamides can form coordination compounds with copper ions in an alkaline environment (pH <7),
A 25% ammonia solution was used to control the pH in order to influence the size and shape of the particles produced by ultra-disperse powders.
As the results of the investigation of the curves of the differential distribution of the particles by size (Figure 3.1) show, the resulting powders are polydisperse with the content of both nanoscale fraction and agglomerates of firmly bound particles, the size of which reaches 10-70 micrometers.
Figure 3.1 -
Histograms of the particle size distribution of powders obtained from copper-ammonia solution at direct current and cathode current density:
a - at j = 0,4 A / cm2;
b - at j = 0,6 A / cm2;
c - at j = 0,8 A / cm2
Figure 3.2 - Histograms of the powder particle size distribution obtained from a copper-ammonia solution in pulse mode, with the length of the pulse and the pause 1: 1 and the cathode density of the current:
a - at j = 0,4 A / cm2,
b - at j = 0,6 A / cm2,
c - at j = 0,8 A / cm2.
Under the same electrolysis conditions, the ultradisperse copper powders produced in the pulse mode have a more homogeneous composition compared to DC powders.
There is a peak in the range of finer dispersion fractions in powders that are produced using water-soluble polymers made from polyvinylpyrrolidone (Fig. 3.3) and polyacrylamide (Fig. 3.4), which is explained by an earlier end to particle growth.
Figure 3.3 - Histogram of the distribution of the particles of the powder obtained with the addition of polyvinylpyrrolidone at the cathode current density j = 0,6 A / cm2:
a - with direct current;
b - in a pulse mode, where the length of the pulse and the pause are 1: 1
Figure 3.4 - Distribution histogram of powder particles obtained with the addition of polyacrylamide and the cathode current density j = 0,6 A / cm2 were obtained:
a - with direct current;
b - in pulse mode pulse length and pause 1: 1.
Results of particle size distribution, ultrafine powders from ammonium chloride electrolytes using different concentrations of stabilizing additives and taking into account the optimal parameters of the extraction, which were determined experimentally and are shown in Table 3.3.
Table 3.3 - Particle size distribution and shape of copper powder
|
Composition No. |
Distribution of the order of magnitude | |||||
| Minimum size, μm. | Nano size fraction content, in%. | Average particle size, µm | ||||
| 1 | 0,026 | 46,3 | 0,9 | |||
| 2 | 0,015 | 68,2 | 0,27 | |||
| 3 | 0,022 | 76,3 | 0,18 | |||
| 4 | 0,019 | 73,2 | 0,56 | |||
| 5 | 0,026 | 51,7 | 0,43 | |||
| 6 | 0,037 | 58,3 | 0,36 | |||
| 7 | 0,033 | 63,7 | 0,31 | |||
| 8 | 0,038 | 56,5 | 0,44 | |||
size distribution
Particles of ultradisperse powders obtained from ammonium chloride electrolytes, which do not contain water-soluble polymers, have the most likely size 26-70 nm with a nanoscale content of not more than 46,3%, and powders of ammonium chloride electrolytes, the Containing water-soluble polymers have the most likely size and higher proportion of nanoscale fractions up to 76,2% and particle content with a smaller size of 7-23 nm at least 15%.
The change in particle size during electrolysis in the presence of water-soluble polymers can be explained by an earlier termination of particle growth due to the formation of complex compounds.
3.2.2 Examination of the quantitative and qualitative composition of the ultradisperse copper powder obtained
Studies of the elemental composition of the ultradisperse powder obtained (Figures 3.5-3.7) showed that the powder obtained by adding water-soluble polymers contains less oxygen (Figure 3.7) than the ammonium chloride electrolyte without polymer injection, it proves that the composition of the Ammonium chloride electrolytes and have a protective effect against the oxidation of air by oxygen.
Figure 3.5 - Elemental analysis of the powder made from copper-ammonium electrolyte without the use of stabilizers.
Figure 3.6 - Elemental powder analysis obtained from copper-ammonia electrolyte with polyacrylamide as a stabilizing agent
Figure 3.7 - Elemental powder analysis obtained from copper-ammonia electrolyte with polyvinylpyrrolidone as stabilizing agent
As can be seen from Figures 3.6 and 3.7, the introduction of polyacrylamide and polyvinylpyrrolidone has a strong influence on the purity of the resulting ultra-disperse powder.
In Fig. 3.5 - the powders made without stabilizers are heavily contaminated with electrolyte products and contain a lot of chlorides and oxides.
The introduction of polyacrylamide reduces the chlorine content to 0,5% and reduces the amount of oxides by 30%. The influence of polyvinylpyrrolidone on the purity of the resulting powder is expressed more than twice by reducing the chlorine content to 0,4% and the amount of oxygen compared to the powder obtained without the addition of stabilizers.
This may be due to the less strong bond between polyacrylamide molecules and the copper surface due to the high molecular weight of the polymer.
This is due to the less strong bond between polyacrylamide molecules and the copper surface due to the high molecular weight of the polymer.
3.2.3 Effect of stabilizers on the particle shape of ultradisperse powder
Influence on the shape and size of the particles of the powder obtained when the polyvinylpyrrolidones are introduced into the electrolyte composition changes the shape of the particles more clearly (Fig. 3.8).
Figure 3.8 - Microphotographs of powders obtained from ammoniacal electrolytes:
- a) pure;
(b) With the introduction of polyvinyl pyrrolidone
Figure 3.8 shows an image of ultra-disperse powders obtained without stabilizing additives.
These powders form rather large agglomerates, on the order of 20-60 micrometers, which are obtained using polyvinylpyrrolidone as a stabilizer, have a greater dispersion and a lower tendency to form agglomerates.
By varying the manufacturing parameters, it was possible to obtain ultra-disperse powders with an increased proportion of particles in the nanometer range. A study of agglomerated ultra-disperse powders has shown that stabilized powders are less robust agglomerates and most agglomerates are destroyed even when subjected to mechanical action. Explained by the chemical bond resulting from contact with unstabilized copper particles is much stronger than the adhesive interaction of stabilized particles, the interface of which is a polymer.
Figure 3.9 - Photomicrograph of copper powder made from ammonia electrolyte without additives
Figure 3.10 - Photomicrograph of copper powder made from ammonia electrolyte with the addition of polyacrylamide
Figure 3.11 -
Photomicrograph of copper powder obtained from ammonia electrolyte with polyvinylpyrrolidone added.
Figures 3.9 - 3.11 show images of copper nanoparticles of electrolytes that are obtained from ammonium chloride (Fig. 3.9) and when water-soluble polymer solutions are added to the electrolyte as stabilizing additives (Fig. 3.9) (310, 3.11). As can be seen from the figures, the introduction of polyvinylpyrrolidone and polyacrylamide into the electrolyte solution reduces the ability to agglomerate and results in the production of particles with a smaller size distribution. As can be seen from Figures 3.9 to 3.11, polyvinylpyrrolidone is the most effective particle stabilizer.
3.2.4 Investigation of the interaction of stabilizers with ultradisperse powder particles
In order to obtain polymer shells on the surface of ultradisperse copper powder, the analysis of the powder was carried out by means of infrared spectroscopy. This method clearly shows the formation of polymeric bonds that form complex compounds on the surface of ultra-disperse copper powder.
Figures 3.12 and 3.13 show data from the investigation of polyacrylamide and copper complex, which were formed in the process of obtaining ultradisperse copper powder.
Figure 3.12 - IR spectrum of an ultra-dispersive copper powder surface, produced with polyacrylamide as a stabilizer
In infrared spectra one can distinguish the valence vibrations of functional groups, which differ from the environment in which the spectra were recorded:
-NH - 3600-3000 см-1
-С-Н - 2900-2750 см-1
С = О -1700-1600 см-1
-С-С - 1300-1200 см-1
As can be seen from the IR spectra shown, the introduction of polyacrylamide in the electrolyte changes the valence vibrations of the amide and carboxyl groups, which indicates the reaction and the maintenance of a copper complex of polyacrylamide.
Figure 3.13 - IR spectrum of an ultradisperse copper powder after removal of polyacrylamide.
As can be seen in Figure 3.13, the complex of polyacrylamide and copper is very unstable, and after a short separation and washing of the powder, it disintegrates and is removed from the surface of the nanoparticles.
This is also confirmed by the thermogravimetric analysis.
Study on ultradisperse copper powder (Fig.3.14), made with polyvinylpyrrolidone as a stabilizer, with a low molecular weight of 8000 ± 1500.
The protective cover formed by this polymer has a stronger chemical bond than that of polyacrylamide.
Figure 3.14 - IR spectra: a - polyvinylpyrrolidone; b - ultradispersed a powder obtained using polyvinylpyrrolidone as a stabilizer.
As can be seen from Fig.3.14b, in contrast to polyvinylpyrrolidone (Fig.3.14a), radiation is adsorbed in the range 2360 - 2200. This indicates the formation of ultradisperse copper powders on the surface, coordination compounds with polyvinylpyrrolidone, which is not removed during separation and electrolyte washing, while retaining its protective properties.
3.2.5 Resistance of stabilized ultradisperse powder to oxidation at high temperatures
Thermogravimetric analysis (TG) was used to test the oxidation and chemical interaction resistance of ultradisperse copper powders. The STA 449C thermal analyzer was used at work.
The analysis was performed in linear heating mode in the range of 10-600 ° C with a heating rate of 10 degrees / min in the air atmosphere.
Figure 3.15 - Thermogravimetric analysis of ultra-dispersed copper powders obtained from ammonia electrolyte without additives
Figure 3.16 - Thermogravimetric analysis of ultra-dispersed copper powders obtained from an ammonia electrolyte with the addition of polyacrylamide
Figure 3.17 - Thermogravimetric analysis of ultradisperse copper powders obtained from an ammonia electrolyte with the addition of polyvinylpyrrolidone
The presented thermograms of ultradisperse copper powders obtained with and without the use of water-soluble polymers as particle stabilizers (Figures 3.15 - 3.17).
The change character of a kind of dependence of thermogravimetric analysis and differential scanning calorimetry on the temperature rise leads to a conclusion about a significant difference in the reactivity of these samples with respect to the oxidation in the air.
Ultra-dispersed powder, which is obtained without the use of stabilizers, starts at 25-140 with little heatingoC oxidize, and the sample weight increases by 1-1,5%.
The intensive oxidation process takes place in the temperature range of 140-300oC instead of
The total weight increase is 14,93%, the further oxidation of the ultra-disperse copper powder takes place with a slight increase in the process speed up to 600оC.
As can be seen on the DSC curve (Fig.3.15), the copper oxidation process is exothermic.
The curve can be divided into 3 parts.
The first section 10-179.3oC exhibits an exothermic reaction which is characteristic of the formation of copper oxide (I), as in Section 179.3-300.5oC can be seen there is a further increase, which can be explained by the transition from copper oxide (I) to copper oxide (II), which fully agrees with the thermogravimetric analysis.
At a temperature section of 300,5оС- 600оС there is a drop and rise in the differential scanning calorimetry curve, which indicates the end of the oxidation reaction and an established equilibrium regime.
The data in Figures 3.15 and 3.16 are identical, which indicates the absence of polymer on the surface of copper particles.
Figure 3.17 shows the curves of thermogravimetry and differential scanning calorimetry in the temperature range 10-600оС for ultra-disperse copper powders that have been stabilized in the process of extracting polyvinylpyrrolidone.
As from the curve of thermogravimetry on the interval 0-160оС can be seen, there is a slight change in mass of 1,5-2%, which can be characterized by the removal of ultradisperse powder from volatile compounds and residual moisture from the surface.
In the temperature range of 160-260оС the weight drop is 13%, which can be explained by the destruction of the polymer and its partial removal from the surface of ultra-disperse copper powder.
In the temperature range 260-340оС shows the thermogravimetry curve a less intense weight loss, which is characterized by the partial removal of the polymer from the surface of ultradisperse powder and the start of oxidation.
At point 340оС on the thermogravimetry curve there is the final removal of the polymeric protective layer and the limited oxidation process of the ultradisperse copper powder, the weight gain on the section of the thermogravimetry curve in the temperature range 340-600оС is 7,92%.
3.2.6 Influence of stabilizers on the phase composition of manufactured ultradisperse copper powders
The phase composition of ultradisperse powders was examined by means of X-ray analysis, the X-ray diffraction diagram is shown in Figure 3.18.
Figure 3.18 - XRF copper EDP spectra: a) without additives;
- b) with the addition of
polyacrylamide;
- c) with the addition of polyvinyl pyrrolidone (PVP).
■- Cu; ♦- Cu2O; ■CuO.
The results of the X-ray phase analysis indicate the presence of crystalline phases of copper and copper oxides in the composition of the examined ultradisperse powders.
In contrast to the starting powder (Fig. 3.18 a), Fig. 3.18 b shows an increased content of the corresponding pure copper phase, as well as copper oxide, but in insignificant amounts.
Figure 3.18c shows the phase composition of ultradisperse electrolyte powders obtained from ammonium chloride with an additional injection of polyvinylpyrrolidone as a stabilizer, which shows the effect of this additive not only on the size of the resulting ultradisperse powders, but also on their structure and properties.
Changes in the phase composition of ultradisperse powders in the manufacturing process indicate that the introduction of polyacrylamide in the electrolyte not only helps to reduce the particle size of ultradisperse powders, but also influences their phase composition.
Also on the X-ray image of Figure 3.18c there are no peaks corresponding to CuO, which indicates the absence of such compounds in this sample.
3.3 Conclusions from Chapter 3
In the course of the investigations carried out on the processes for obtaining powders from ammonia electrolytes due to the generation of reducing agents at the anode and on the basis of the experimental data obtained, the following conclusions were drawn:
1) The possibility is shown to control the properties of ultradisperse powders, as particle shape, phase and the particle size distribution in the process.
2) The production of ultradispersed powders in the pulse mode makes it possible to reduce the size of the particles and to achieve a more uniform distribution over the particle size distribution, relative to ultradisperse powders which are produced with direct current.
3) It was found that the introduction of polyacrylamide in the electrolyte reduces the average particle size to 0,36 µm and increases the amount of the nanofraction. A polymer film forms on the surface of the particles, which is removed from the surface together with the electrolyte during washing, which improves the purity of the resulting ultradisperse powder. The shape of the resulting powder is dendroid.
4) The introduction of polyvinylpyrrolidone in the electrolyte reduces the size of the particles, changes the particle size and the fraction composition of the resulting powder, offers additional protection against oxygen, which reduces its amount by about two times, and prevents the occurrence of copper oxides in the end product (II ).
5) Powders that were produced using polyvinylpyrrolidone as stabilizer particles are only thermally oxidized after removal from the surface of the protective film.
6) Copper powder stabilized with water-soluble polymers can be recommended as an alloy additive for use in anti-friction composites based on various polymer matrices to increase hardness and reduce wear.
Chapter 4
Investigation of the properties of composite materials filled with ultra-disperse copper powder
4.1 Composites with a fluoroplastic matrix
When developing composite materials with a polymer matrix, the processes that occur in the mixture must be taken into account. These are phase transitions of the material (glass transition, softening, plasticization) and difficulties in product formation due to rheological properties and the partial destruction of macromolecules in polymer chains.
4.1.1 Optimization of composite material compositions using the method of the mathematical planning experiment
The method of central composition planning was used to optimize compositions [132]. The method is chosen as influencing factors:
X1 - Concentration of ultra-dispersed copper powder, X2 - pressure, X3 - Mixing time.
Destructive tension during compression is accepted as a reaction function. The levels and intervals of the variation are given in Table 4.1.
The following basic levels and factor variation intervals were selected in order to implement planning matrices for the experiment to optimize the reception conditions for obtaining the compositions. Materials based on ultra-dispersed copper powder (Table 4.1).
Table 4.1 - Values of the basic level and variation intervals
Factors of the experiment planning matrix to optimize the conditions for the production of copper powder
|
factor
|
Variations interval | Basic level | |
|
Name
|
Illustration |
||
|
Ultradisperse powder content in the mixture,% by weight |
X1
|
20 | 50 |
| Pressure, MPa
|
X2
|
40 | 180 |
| Mixing time, minutes
|
X3 |
5 | 10 |
The choice of such values was determined by preliminary experiments and represents an average interval of the variation of the factors.
One of the main factors in the production of nanomaterials is the mixing time of the mixing components, which is responsible for the even distribution of the filler over the entire volume of the matrix.
In order to optimize the conditions for the extraction of a composite material, a planning matrix for a full factor experiment 23 was implemented.
(Table 4.2).
Table 4.2 - Experimental planning matrix to optimize the conditions for nickel powder production
|
No experiment
|
Factor values in coded variables |
Y |
|||||
| X1 | X2 | X3 | X1X2 | X1X3 | X2X3 | ||
| 1
|
+1 | +1 | +1 | +1 | +1 | +1 | 292 |
|
2 |
-1 | +1 | +1 | -1 | -1 | +1 | 251 |
|
3 |
+1 | -1 | +1 | -1 | +1 | -1 | 210 |
|
4 |
-1 | -1 | +1 | +1 | -1 | -1 | 180 |
| 5
|
+1 | +1 | -1 | +1 | -1 | -1 | 286 |
| 6
|
-1 | +1 | -1 | -1 | +1 | -1 | 241 |
| 7
|
+1 | -1 | -1 | -1 | -1 | +1 | 163 |
| 8 | -1 | -1 | -1 | +1 | +1 | +1 | 123 |
The material's resistance to breaking loads was used as a reaction function.
To estimate the spread of reproducibility, the experience at the base level was repeated 3 times.
Based on the results obtained, the coefficients of the quasilinear regression equation (4.1) were calculated:
 The signs of the coefficients obtained mean that the movement should take place up to the required maximum in the direction of increasing the concentration of ultradisperse copper powders, the pressing pressure and the mixing time.
The signs of the coefficients obtained mean that the movement should take place up to the required maximum in the direction of increasing the concentration of ultradisperse copper powders, the pressing pressure and the mixing time.
This means that in order to achieve the maximum strength of the material, it is necessary to create conditions that prevent agglomeration of the filler.
The studies carried out beyond the planning matrix have shown that an increase in the mixing time of the composite material components does not lead to improved properties, so that instead of the variable X3 take the optimal mixing time of 12,5 minutes.
Examination of the range of optimal conditions
In order to optimize the planning matrix, a central compositional planning of the experiment was carried out.
Let's create a planning matrix with the two most important factors (Table 4.3).
Table 4.3 - Values of the baseline level and intervals of variation of the factors of the planning matrix of the experiment for studying the range of optimal conditions for the extraction of copper powder
|
factor
|
Variations interval | Basic level | |
| designation
|
Symbol
|
||
| Ultradisperse powder content in the mixture, percent by weight.
|
X1 |
20 | 50 |
|
Pressing pressure, MPa |
X2 |
50 | 150 |
In order to optimize the conditions for the production of composite material, a planning matrix for a full-fledged experiment with additional investigations in the "star" points and the plan center was implemented (Table 4.4). (Table 4.4).
Table 4.4 - Experiment planning matrix for optimizing the conditions for the production of nickel powder
|
Experiment number
|
Factor values in coded variables | Y | |||||
| X1 | X2 | X1X2 | X1* | X2* | |||
|
Complete factor experiment
|
1 |
-1 | -1 | +1 | +0,33 | +0,33 | 180 |
|
2 |
+1 | -1 | -1 | +0,33 | +0,33 | 210 | |
|
3 |
-1 | +1 | -1 | +0,33 | +0,33 | 251 | |
|
4 |
+1 | +1 | +1 | +0,33 | +0,33 | 292 | |
|
Experiments at star points |
5
|
+1 | 0 | 0 | +0,33 | -0,67 | 203 |
| 6 | -1 | 0 | 0 | +0,33 | -0,67 | 306 | |
|
|
7 | 0 | +1 | 0 | -0,67 | +0,33 | 286 |
| 8 | 0 | -1 | 0 | -0,67 | +0,33 | 298 | |
| Experience at the heart of the plan | 9 | 0 | 0 | 0 | -0,67 | -0,67 | 296 |
Based on the results obtained, the coefficients of the quasilinear regression equation (4.3) were calculated:
(4.3)
Higher filler concentration and higher pressing pressure contribute to higher material loads.
The answer areas are shown in Figure 4.1.
Figure 4.1 - Response area
The optimal content of ultra-disperse copper powders is 45-47% of the mass, and the pressing pressure is 173,6 MPa.
The ultradisperse powder obtained by the electrolysis method, stabilized by water-soluble polymers of the chloride-ammonium electrolyte, was used as an additive to polymer composite materials with a high degree of filling of 30-70%.
Ultradisperse copper powders were used as fillers for polymers when the F-4 and PE-277 were selected because of their high sliding properties, but insufficient physical and mechanical properties, and the similarity of the processing and production methods of the products used in powder metallurgy.
A mathematical model was created to investigate the influence of nanoscale copper particles, which were obtained by various methods described in Chapter 3, on the properties of composite materials, which enables the composition of composite materials based on fluoroplastic F-4 to be optimized. The optimized data are shown in Table 4.5.
Table 4.5 Compositions of mixtures for the preparation of samples based on fluoroplastic F-4, optimized by the method of mathematical planning.
|
No. compositions
|
Component content, mass,%
|
|||
| PTFE 4
|
Powder obtained without stabilizers
|
Powder obtained by adding polyacrylamide
|
Powder obtained by adding polyvinylpyrrolidone
|
|
| 1
|
100 | - | - | - |
| 2 | 65 | 35 | - | - |
| 3
|
65 | - | 35 | - |
|
4 |
65 | 35 | ||
|
5 |
54 | 46 | ||
|
6 |
54 | 46 | ||
| 7
|
54 | 46 | ||
| 8
|
37 | 63 | ||
|
9
|
37 | 63 | ||
|
10
|
37 | 63 | ||
In order to obtain reliable data, 3 samples of each composition were prepared.
4.1.2 Deformation of sintered composites
The mixing was carried out in a drum mixer with ceramic balls of 10 mm in diameter for 12-15 minutes at a peripheral speed of the drum of not more than 0,3-0,5 m / s.
It was pressed at a pressure of 1100-1200 kg / cm2 in a cylindrical shape d = 12 mm.
After deburring, the linear dimensions of the samples obtained before and after sintering were measured with the USSR 5417 micrometer (Table 4.6).
Sintering was carried out at 370-410 ° C for 2,5 hours in an argon inert gas environment.
Table 4.6 - Change in sample linear sizes during sintering
|
Sample number
|
Before sintering
|
After sintering
|
Change in linear dimensions | ||||
| hmmmm | d, mm | hmmmm | d, mm | Δh, mmm | Δd, mmm | ||
| 1 | 6,65 | 12,01 | 6,31 | 11,53 | 0,34 | 0,48 | |
| 2 | 6,35 | 12,01 | 6,17 | 11,69 | 0,18 | 0,32 | |
| 3 | 6,48 | 12,0 | 6,32 | 11,73 | 0,16 | 0,27 | |
| 4 | 6,93 | 12,0 | 6,71 | 11,71 | 0,22 | 0,29 | |
| 5 | 6,17 | 12,0 | 6,01 | 11,75 | 0,16 | 0,25 | |
| 6 | 6,28 | 12,0 | 6,14 | 11,78 | 0,14 | 0,22 | |
| 7 | 6,34 | 12,0 | 6,19 | 11,8 | 0,15 | 0,2 | |
| 8 | 6,69 | 12,0 | 6,43 | 11,73 | 0,26 | 0,27 | |
| 9 | 6,21 | 12,0 | 5,97 | 11,76 | 0,24 | 0,24 | |
| 10 | 6,37 | 12,0 | 6,09 | 11,81 | 0,28 | 0,19 | |
By changing the linear dimensions of the samples, it can be seen that the samples made of composite materials that are alloyed with copper powder shrink less than in height and diameter.
4.1.3 Physico-mechanical properties of composite materials
Pressure tests were carried out to determine the effect of ultra-disperse copper powders on the strength properties of composite materials. The data are shown in Table 4.7.
Table 4.7 - Compression test data for composite materials
| Composition number
|
Compression Destructive tension kgs / cm2
|
|
| 5% deformation | 10% deformation | |
| 1
|
100 | 125 |
| 2
|
218 | 283 |
| 3
|
224 | 296 |
| 4
|
251 | 360 |
| 5
|
278 | 334 |
| 6
|
286 | 349 |
| 7 | 302 | 358 |
| 8
|
286 | 309 |
|
9 |
274 | 312 |
| 10 | 292 | 318 |
The above data therefore show that the use of ultra-disperse copper powders as an additive to highly filled composite materials increases their resistance to the applied load. In addition, the addition of stabilized ultra-disperse copper powders obtained from an anodically synthesized electrolyte showed a higher result than the use of branded powders, which come from the same technology, but without stabilizers. It should also be noted that due to the relaxation properties of the polymers and the even distribution of the filler over the entire volume, it was found that if the deformation during compression does not exceed 4%, materials that are up to 45-57% stabilized, ultradisperses Copper powders contain, after removal of the load, are able to restore their previous size and shape without losing their anti-friction properties. Materials made with unstabilized ultradisperse powders do not have as strong properties to maintain their shape and with a 1-1,5% deformation they cannot restore their original shape.
This is due to the adhesive force between the matrix and the filler.
This is achieved by stabilizing the filler and protecting it from agglomeration and various influences (e.g. high temperatures and oxidation).
An important role in this process is played by polyvinylpyrrolidone, which is used as a stabilizer, in the production of ultradisperse powders and in the formation of polyvinylpyrrolidone films on the surface of nanoparticles, which in turn enables the use of such powders for the production of products from highly filled polymer composites. The properties of such compositions can differ significantly from those achieved by the introduction of various unstabilized fillers. Of particular interest are nano and micro metal particles, in this case copper. The effect of various additives on the hardness of the composite materials obtained was investigated.
As from Figure 4.2. it can be seen that the introduction of stabilized ultradisperse powder significantly improves the properties of composite materials.
At the same time, the increase in material hardness was observed in the samples obtained on the basis of the fluoroplastic F-4.
Figure 4.2 - Brinell hardness of uncured samples
Depending on the cooling rate after sintering, it is possible to obtain hardened products with higher hardness, for this purpose the sample after sintering with 100-200oC / sec cooled down quickly. This is probably due to the reduced crystallinity of the polymer and hence its density and hardness.
Figure 4.3 - Brinell hardness of the samples after hardening
The introduction of ultra-disperse copper powders leads to an almost linear hardening of the material.
As can be seen in Figures 4.2 and 4.3, the introduction of copper nanoparticles more than doubles the hardness of a composite compared to a pure polymer. Compositions 7 and 10 correspond to the maximum hardness value compared to other compositions.
However, increasing the filler content to 70% leads to a decrease in hardness. This is due to the fact that with a filler content of 70% or more, the pressing pressure has to be increased, but the structure of the composite materials deforms and forms agglomerates, which leads to a regression of the properties of composite materials.
4.1.4 Anti-friction and wear-resistant properties
When choosing the optimal filler content, it should be noted that the materials used for plain bearings must have high wear resistance at high specific loads and thus a low coefficient of friction.
To assess the wear resistance of the developed composite materials, the dependencies of the coefficient of friction on the specific load and the linear dry friction wear were determined using the methods described in Section 2.4.2. The test results are shown in Figures 4.4-4.6.
Figure 4.4 - Dependence of the coefficient of friction on the dry load
Figure 45 - Dependence of the coefficient of friction on the dry load
Figure 4.6 - Dependence of the coefficient of friction on the dry load
Analysis of the data obtained shows that the introduction of ultradisperse copper powders into the fluoroplastic matrix promotes an insignificant increase in the coefficient of friction at low loads, and the coefficient of friction decreases as the load increases.
The payload of the developed composite material increases by more than 30%.
The introduction of ultra-disperse copper powders stabilized by water-soluble polymers, even with a small fill, help reduce the coefficient of friction and increase the workload of composites by 30 to 50% relative to the relatively pure polymer, and composites that are unstabilized Ultradisperse copper powder contain about 10 to 15%.
Tests have also been carried out on CM friction in oil.
The results of the tests are shown in Figures 4.7-4.9.
Figure 4.7 - Dependence of the coefficient of friction on the load in MS-20 oil
Figure 4.8 - Dependence of the coefficient of friction on the load in MS-20 oil
Figure 4.9 - Dependence of the coefficient of friction on the load in MS-20 oil
It should be noted that the friction coefficient in the oil is much lower compared to the friction in the dry, in addition to the lubricating effect, the oil provides thermal regulation of the samples.
When high loads are reached, oil can be pushed out of the friction zone, which threatens to increase the coefficient of friction and wear. For this reason, the recommended permanent operating loads should not exceed 5,5-6 MPa for metal-polymer composites, although short-term loads can reach 12-14 MPa.
One of the goals when introducing the investigated additives is increased resistance to wear.
Data on the influence of imported ultradisperse copper powder on weight and linear wear are shown in the form of diagrams (Figures 4.10-4.11). The wear resistance was determined in pairs with steel (steel 45), test times of 1, 2 and 4 h at 4 MPa friction dry load according to the method described in chapter 2.2.
Figure 4.10 - Dependency of the weight wear of composite materials on the additive inputs at Pd = 4,0 MPa for 1, 2 and 4 hours
Figure 4.11 - Dependence of the linear wear of composites on additive inputs at Pd = 4,0 MPa for 1, 2 and 4 hours
The increased wear in the initial phase is due to irregularities both on the specimen and on the surface of the counter body, which in turn leads to high wear in the initial phase of the test, but the wear is markedly reduced after the irregularities have been compensated for. This happens when, under the influence of high pressures and temperatures, decomposition products and filler particles move from the sample surface to the counter surface, forming perfectly adapted levels and there is an even distribution of the specific loads over the entire surface of the friction body, which both reduces the coefficient of friction and also explains the wear. Figures 4.12 and 4.13 show the sample surfaces before and after tests on a friction machine, the study was carried out on a scanning probe microscope SolverHV.
Figure 4.13 - Surface profile of the specimens after friction
As can be seen from Figure 4.12, the deviation of the surface profile in the examined section before the tests is about 0,3-0,35 µm.
The data from the surface study after friction are presented in Figure 4.13.
Most of the irregularities on the friction surface have been smoothed or filled with particles caused by the wear of composite materials or the counter body, the deviation from the center line is 0,08-0,12 µm.
This also reduces wear and friction coefficients after surface treatment.
4.1.5 Investigation of the structure of composite materials
One of the most important processes in the production of composite materials is the mixing of components: the more homogeneous the composition of the material, the higher its physical and mechanical properties (hardness, wear resistance).
When mixing the mixture of composite materials, high homogeneity is almost impossible with unstabilized ultradisperse copper powders due to the aggregation of the filler particles. If stabilized fillers are used in the stirring process, the resulting agglomerates are dispersed again. It is also possible for two opposing processes of formation and destruction to be balanced at a certain point in time.
In this case there is no point in continuing to mix because the quality of the mixture does not change. According to studies, the greatest homogeneity in the distribution of stabilized filler particles is achieved after 12-15 minutes of mixing time, while for unstabilized fillers this time is 40-45 minutes, which is at least three times. In order to assess the quality of the mixture, microstructural studies were carried out on composite materials with various fillers, which were obtained by cold pressing with subsequent sintering (Figure 4.14 (ag)
Figure 4.14 - Microstructure of composite materials with different fillers at 250x magnification.
(a) copper powder obtained without the use of stabilizers with an average particle size of 1,92 µm;
- b) copper powder obtained without the use of stabilizers with an average particle size of 1,27 μm;
(c) copper powder obtained using polyacrylamide and medium particle size stabilizers 0,54 µm;
- d) copper powder obtained using polyvinylpyrrolidone and an average particle size of 0,46 µm. Figure 4.14 (a and b) shows that a composite material that contains ultradisperse copper powder without stabilizers forms large agglomerates, which leads to an uneven distribution of the particles over the entire volume of the polymer matrix and in turn does not allow the material that the ultradisperse powders to realize inherent alloy properties. Figure 4.14 (c) shows an image of a composite material that contains ultra-dispersed copper powder as a stabilizer in the process of polyacrylamide production, which significantly reduces its ability to agglomerate,
In the course of examining the structure of the composite material obtained with the application, however, there are still unfilled areas of the polymer, which also affects the unevenness of the physical and mechanical properties.
Figure 4.14 (d) shows
an image of the structure of a composite material obtained using ultradisperse powder derived from an ammonia solution containing polyvinylpyrrolidone as a particle stabilizer.
The figure shows that the filler is evenly distributed, there are no deficiency or excess zones of the filler in the entire polymer volume, this makes it possible to maximize the potential of ultradisperse powder as filler for F-4.
Figure 4.15 - Interaction between the filler and the metal polymer matrix
Composites based on F-4 and UDP copper:
(a) obtained without stabilizers in direct current;
- b) obtained without stabilizers in pulse mode;
- c) use of polyacrylamides as stabilizers;
- d) Use of polyvinylpyrrolidone as a stabilizer.
When examining the influence of stabilized ultradisperse powder on the structure of composite materials, it was found that there is no interaction between the polymer matrix and the filler when using unstabilized ultradisperse powder, which explains the large phase boundary between the filler and the polymer.
Studies of F-4-based composites with stabilized ultradisperse copper powders (Figure 4.15 g) showed that after sintering, the interface zone between the polymer and the filler is reduced, which is the result of a high adhesion interaction and leads to improved material properties.
At the same time, the reduction of the interphase zone is observed both with medium filler concentrations (40-60%) and with highly filled composite materials (more than 60%).
4.2 Development of composite materials using polyethylene as a matrix.
In this work, in addition to PTFE-4, polyethylene-277 was also used as the polymer matrix for the development of composite materials, and fillers were also ultra-disperse copper powders, which were produced using various water-soluble polymers as stabilizers.
A number of compositions have been developed in the course of research, the optimal compositions of which are given in Table 4.7.
Table 4.7 - Composition and properties of the compositions studied
|
Composition number
|
Component content, mass,%
|
|||
| Polyethylene 277
|
Powder obtained without stabilizers
|
Powder obtained by adding polyacrylamide
|
Powder obtained by adding polyvinylpyrrolidone
|
|
| 1 | 100 | |||
| 2 | 90 | 10 | ||
| 3 | 90 | 10 | ||
| 4 | 90 | 10 | ||
| 5 | 80 | 20 | ||
| 6 | 80 | 20 | ||
| 7 | 80 | 20 | ||
| 8 | 70 | 30 | ||
| 9 | 70 | 30 | ||
| 10 | 70 | 30 | ||
4.2.1 Anti-friction and wear-resistant properties of composite materials
In this report, for the purpose of evaluating the performance of the developed composites, the dependencies of the coefficients of friction on the value of the specific load as well as linear wear due to dry friction were determined. The results of the coefficient of friction tests are given in Figures 4.16-4.19.
Figure 4.16 -
Dependence of the coefficient of friction in the dry
Figure 4.17 -
Dependence of the coefficient of friction in the dry
Figure 4.18 - Dependence of the coefficient of friction under dry conditions
Analysis of the data obtained shows that the introduction of polyethylene as
Ultradisperse copper powder filler stabilized with water-soluble polymers of polyacrylamide and polyvinylpyrrolidone helps to reduce the coefficient of friction, and as a result, the wear resistance of the resulting composite material depends.
Reliability and durability of parts made of developed composite materials are largely determined by the size of the weight and linear wear and tear.
The definition of linear wear is shown in the following figure 4.19
Figure 4.19 - Linear dependence of additives on wear
As can be seen from the data presented, the introduction to the composition of the Liede stabilized polyvinylpyrrolidone copper powder reduces linearity and wear compared to composite materials that contain unstabilized ultradisperse powders prone to agglomeration. The developed composite materials can be used for parts that work in friction units without lubricants or moisture in the friction zone. The decisive influence on the physical and mechanical properties of these materials has an interphase interaction in the contact zones of heterogeneous phases.
4.2.2 Influence of ultra-disperse copper powders on the hardness of composite materials
The effect of additives incorporated in the compositions on the hardness of the samples obtained was investigated.
The study was carried out on the AS-111 device according to the methodology presented in Chapter 2. The results are shown in Figure 4.20.
Figure 4.20 - Brinell hardness dependence of the samples on the additives introduced
As can be seen in Figure 4.20, the hardness of the material increases with the introduction of ultra-disperse copper powders.
However, the introduction of copper particles, which are obtained when introduced into the electrolyte as a stabilizer of the polyacrylamide, also increases the hardness of the materials obtained (about 1,5-2%) and differs from the material used when using ultradisperse powders is obtained without stabilizers.
The best properties for this are shown by the materials obtained with ultradispersed powders made from stabilized polyvinylpyrrolidone. Obviously this is achieved by the even distribution of the particles. Ultradisperse powders in the entire polymer volume, without creating areas with high filler concentration or deficiency, as is the case with unstabilized ultradisperse powders.
4.2.3 Influence of ultra-disperse copper powders on the strength of composite materials
The influence of ultra-disperse copper powders on the pressure resistance of the developed composite was examined.
The results of the investigation are shown in Table 4.8.
Table 4.8 - Strength properties of composite materials.
|
No sample
|
Compressive strength, Kgs / cm2 | |
| Deformation 5% | Zerstörung | |
| 1 | 125 | 131 |
| 2 | 140 | 152 |
| 3 | 147 | 155 |
| 4 | 156 | 164 |
| 5 | 164 | 171 |
| 6 | 170 | 183 |
| 7 | 189 | 202 |
| 8 | 183 | 198 |
| 9 | 186 | 205 |
| 10 | 203 | 226 |
As can be seen from the table, the influence of a filler with a branched dendritic nanostructure has a high influence on the strength of the material even with low filling.
However, studies show that the introduction of ultradisperse copper powder, which is stabilized with a polymer shell made of polyvinylpyrrhoids, contributes to the physical and mechanical properties of composite materials due to the interaction at the molecular level and the formation of bonds with the surface of the ultradisperse copper powder.
4.2.4 Examination of the surface layer structure
In the context of the work, the structure of the surface layer of the composite material as well as the surface of the material and the counter body were examined before and after the friction tests.
The results of the research made it possible to explain the reduction in the coefficient of friction and the high wear intensity in the first hours of the test.
The decrease in the coefficient of friction with increasing load is explained by the fact that, due to the unevenness of the material, the zone of actual material contact deviates from the theoretical zone by several orders of magnitude. As a result, ultra-high pressures and temperatures develop at the peaks, which have a negative effect on the coefficient of friction of the composite material. With increasing load, the peaks of the unevenness on the material are smoothed, the contact area of the materials increases, the pressure is distributed evenly over the entire surface, the temperature stabilizes, which leads to a reduction in the coefficient of friction.
Figure 4.21 shows photos of the surface of composite materials based on PE-277 before and after friction at 400x magnification.
Figure 4.21 - Material surface:
- a) before friction;
- b) after the friction
There are copper particles on the surface of the sample that have been transferred to the counter body by friction.
(Figure 4.22).
The surface was smoothed and the wear products filled in the irregularities. Based on this data, the decrease in the coefficient of friction can also be explained in this case.
Figure 4.22 - Counter body surface after friction
Examinations of the counter body surface, which were carried out with the energy-dispersive X-ray fluorescence spectrometer, have shown that after the friction a thin copper film forms on its surface, which is involved in the selective transmission and contributes to the improvement of the tribotechnical properties.
After the friction tests, an elemental analysis of the counter body surface was carried out, the results of which are shown in Figure 4.23.
Figure 4.23 - Elemental analysis of the opposing body surface
The data shown in Figures 4.21-4.23 show that the friction pair metal-polymer-composition-steel can exhibit the phenomenon of no wear due to the formation of a servo whiting film. * (lat. servo-witte)
The mechanism of the formation of the servo white film depends on the lubricant, the materials involved and the friction conditions.
The metal film that forms during friction is called a “servo winding”.
The mechanism of forming a servo wrap film on friction surfaces can be different. Since the copper layer formed on the surface becomes thinner due to its transfer to a steel surface, the polymer surface is further destroyed.
Since the copper layer formed on the surface becomes thinner due to its transfer to the steel surface, the polymer surface is further destroyed. This process continues until a 0,01 to 2 micron thick copper layer is formed on both steel and polymer surfaces.
As soon as the copper film has coated the polymer and steel surfaces, the copper molecules can no longer break out of the polymer, the process of polymer destruction is stopped and an established system of selective deferral comes into effect. The process of forming a servo white film on a steel surface is discreet. The copper particles from the polymer surface are transferred to the top of the unevenness of the steel surface of the counter body, ie the parts made of steel that come into direct contact with copper. Then there is a gradual “slipping” of the accumulated copper into the cavity of the irregularities.
During the friction, the oxide layer on the steel surface is destroyed (the surface is restored), which ensures high adhesion of the copper film to the steel surface. As a result, the latter is coated with a copper film, and a pair of friction polymer steel becomes a pair of copper-copper.
When analyzing the data obtained, it can be said that the introduction of ultradisperse copper powder into the composition of copper contributes to the hardness of the samples made of developed materials compared to pure polyethylene.
Figures 4.22 and 4.23 show the sample surfaces before and after
Tests on a friction machine, the study was carried out on a scanning probe microscope SolverHV.
Figure 4.25 - Sample surface after the grater test
As you can see in Figure 4.24, the sample surface has a complex topography, the peaks of peaks and valleys with a height of 0,1 to 1,1 micrometers can be seen all over the surface.
The surface of the sample after the rubbing machine test, as shown in Figure 4.25, has a softer relief without sharp peaks of bumps or depressions. After tribo tests, small elevations can be seen on the surface, which can be attributed to the fact that the copper particles involved in the selective transmission began to form a servo winding film.
4.3 Conclusions on Chapter 4
In the course of the investigations carried out on the processes for obtaining anti-friction composite materials with a polymer matrix, the factors which influence the properties of composite materials were investigated, and the following conclusions were drawn on the basis of the experimental data obtained:
- The developed mathematical model for optimizing the composition of composite materials makes it possible to determine the influence of technological parameters on the physical and mechanical properties of composite materials and to predict their properties.
- The influence of a filler on the change in linear sizes is examined in the sintering of composite materials. It has been found that linear dimensions of materials alloyed with stabilized copper powder have undergone less shrinkage, both in height and in diameter.
- It has been found that the even distribution of the filler over the entire volume of the polymer matrix is only achieved through the use of stabilized ultradisperse copper powder, which also affects the homogeneity of the properties over the entire volume of the composite material and increases properties such as compressive strength Hardness, wear resistance and increased maximum operating loads of more than 30% compared to unstabilized fillers.
The use of ultradisperse powders stabilized with polyvinylpyrrolidone made it possible to increase the mixing time and to achieve a more uniform distribution of the particles in the matrix volume of the composite material.
- It has been found that the use of stabilized ultra-disperse copper powders reduces the interphase boundary between the matrix and the filler surface, which increases the adhesion of the filler to the polymer matrix.
- Examination of the structure of the surface layer of the composite and the counter body before and after the friction tests showed that the friction of the material produces an effect of selective transfer of copper to the counter body surface, which results in a more uniform surface of the conjugated friction surfaces, which reduces wear.
- The introduction of more than 30% polyethylene filler affects the melt viscosity, which complicates the processing of the material by extrusion and injection molding, as well as the increase in temperature when the mixture is processed and the partial destruction of the polymer.
Conclusions and conclusions
Depending on the chemical nature of the nanoparticles, the structure on the friction surface changes due to agglomerates of dispersed particles.
When used as a filler, locally unstabilized ultradisperse copper powders, which are rarely found on the friction surface, form micron particle clusters that form irregularities from 1,0 to 1,2 micrometers in height.
The introduction of the same amount of stabilized ultradisperse powders in F-4 leads to the formation of a surface layer of the sample with an even distribution of the filler particles.
In this case, the area occupied by clusters is 3 times larger than the area of the microparticle localization sites. The decrease in wear of composites containing ultradisperse powders stabilized by water-soluble polymers by 1,5 to 2 times indicates greater resistance to contact deformation of the surface layer of the sample compared to composites that contain unstabilized ultradisperse Powder included.
The coefficient of friction of composite materials has dropped 1,7 times, which is obviously due to the optimal roughness of the friction surface.
And the following conclusions about the work were also drawn:
- The developed method for the production of ultradisperse copper powders based on electrolysis makes it possible to change the particle shape, the chemical activity and the phase composition. Such as changing the concentrations of water-soluble polymers and salts in the electrolyte and varying current parameters.
2) The use of polyacrylamide as a stabilizer can reduce the average particle size up to 0,36 μm by increasing the number of nano fraction. Due to the formation of a polymer film on the surface of the particles, which together with the electrolyte in the process of washing with water, thereby improving the purity of the resulting ultra-dispersible powder. The shape of the powder is a dendrite.
3) The introduction of polyvinylpyrrolidone in the electrolyte reduces the average particle size to 0,18 μm, provides additional protection against oxygen by reducing its amount by about two times, due to the formation of a polymer film on the surface of the copper particles, which after the separation and washing of the electrolyte remains on the powder and prevents the occurrence of copper oxides in the end product (II).
4) The inclusion of powders in pulse mode allows the size of the particles produced by ultra-dispersed powders to be reduced, which in combination with the addition of water-soluble polymers of polyacrylamide and polyvinylpyrrolidone in the electrolyte enables a smaller particle size distribution. The smallest particle size variation is achieved when polyvinylpyrrolidone with a concentration of at least 6 g / l in pulsed mode with a current cathode density of 0,6A / cm2 is injected into the electrolyte.
5) Stabilized water-soluble copper powders can be recommended for use in anti-friction composite materials based on various polymer matrices as an alloy additive to increase hardness and reduce wear.
6) The developed mathematical model to optimize the composition of composite materials makes it possible to determine the influence of technological parameters on the physical and mechanical properties of composite materials and to predict their properties.
7) The influence of a filler on the change in linear sizes during sintering of composite materials is investigated. It is found that the change in linear sizes of materials alloyed with stabilized copper powders occurs to a lesser degree in height and diameter.
8) The use of ultradisperse copper powders stabilized with polyvinylpyrrolidone makes it possible to reduce the mixing time and to achieve a more even distribution of filler in the entire polymer matrix, reduces the phase interface between the matrix and the filler, which increases the adhesive strength of the filler on the polymer matrix elevated. The use of ultradisperse copper powders stabilized with polyvinylpyrrolidone increases the physical and mechanical (hardness, pressure and wear resistance) and anti-friction properties (coefficient of friction) of composite materials by more than 30% in relation to unstabilized nano-fillers.
9) The effect of the selective transfer of copper atoms to the counter body surface was found, which creates a more uniform surface, and thus a more even distribution of loads over conjugate friction surfaces, which reduces the coefficient of wear and friction.
10) The stabilization of the ultradisperse copper powder particles enables them to be introduced into the polyethylene matrix as a filler by 18 to 27% with a slight change in the viscosity of the melt, which enables the material to be processed by extrusion and injection molding and leads to faster heating of the processed mixture.
List of abbreviations and symbols
PVP with polyvinyl pyrrolidone;
PAA-polyacrylamide;
XRF - X-ray phase analysis;
DTA - differential thermal analysis;
t - time;
SEM - scanning electron microscopy;
Surfactant - surfactant;
j - current density;
T - temperature;
PE 277 is polyethylene;
F-4 - Teflon-4;
LDPE - low pressure polyethylene;
SVC - ultra high frequency;
UDP - ultra-disperse powder;
KM - composite material;
ТМА - thermomechanical analysis;
TG - thermogravimetry;
DSC - Differential Scanning Calorimetry;
IR - infrared;
f - coefficient of friction;
N is the moment of friction;
Rud - wing loading;
N - friction surface;
PI - selective transmission;
CPS - static cold pressing;
XA - ammonium chloride
Bibliography:
See in the original Russian text.
Institute of rare earth metals
We apologize for grammatical errors that arise from the translation from Russian. Our employee is not a professional translator.
Read the full Dissertation on Making Ultra Fine Copper Powder - PDF
German:
PRODUCTION OF ULTRADISPERS COPPER POWDERS, STABILIZED WITH WATER-SOLUBLE POLYMERS FOR ANTIFRICTION METAL POLYMER MATERIALS.
Original in Russian: