Production of high-grade electrolytic metal powders
The example of electrolytically produced cobalt powder, nickel powder and copper powder.
Soluble and insoluble anodes connected to separate current sources are used to control the dissolution of the soluble anode during the electrolysis process and the concentration of metal ions in solution by correcting the ratio of the anode strengths of the soluble and insoluble anodes at constant cathode density Control electricity. In addition, the constant value of the cathode density of the current is achieved by the constant area of the cathode and the sum of the current on the soluble and insoluble anodes.
EFFECT: To avoid labor-intensive operations in the correction of electrolytes by balancing cathode and anode current outputs in the production of metal powders.
The claimed technical solution relates to the field of powder metallurgy, in particular to methods for metal powder by electrolysis.
The recovery of electrolytic powders from metals is based on the electrolysis of aqueous solutions containing a salt of the metal and buffering additives. 1] (kudra O., Hitman E., Electrochemical Production of Metal Powders, Kiev, 1952).
During the process of electrolysis in the solution of the change in the initial concentration of substances, which leads to a change in the conditions to obtain a powder. This fact creates a problem in the industrial application of this method in the production of metal powders in labor-intensive operations adapting to the composition of the electrolyte. The main reason for changing the concentration of substances in solution is the difference in cathode and anode output current values. All the more so as the anodic dissolution of some metals causes no difficulties and they dissolve easily without any sign of passivation. In the meantime, in addition to the recovery of metal ions on the cathode in powder form, as a rule, the side effect is recovery of hydrogen ions. The excess anode output via the cathode output affects the accumulation during the electrolysis process of the dissolved metal cations. Since maintaining a constant electrolyte composition is an indispensable prerequisite for obtaining powders with desired properties, when electrolysis is necessary to organize a controlled dissolution of the anodes.
Known method for producing metal powders by electrochemical methods [2] (USSR Author's Certificate No. 70696, priority date 08.02.1947 g) consisting of powder electrodeposition of metal on a horizontally arranged cathode using soluble or insoluble anodes vertically around the cathode on their sides or above.
In another known process, the electrolytic production of highly dispersed magnetic powder-cobalt [3] (USSR Author's Certificate No. 189155, priority date 17.11.1966 g), which results in the electrolysis of a cobalt-containing electrolyte using cathodes made of steel, copper and other metals and anodes Cobalt (soluble) or lead (insoluble).
The disadvantage of this method is that when replacing the soluble insoluble anodes in the electrolysis process, the depletion of the solution of metal ions (main component) and thus the complete adjustment of the deposition of metal powder. Therefore, these methods require time-consuming adjustments in the composition of the electrolyte cations of metals.
The most technically closest and therefore prototyped SPO relates to improving the processes for obtaining pure metal cobalt powder [4] (British Patent No. 403.281, priority date 29.04.1932 year), according to which the electrolytic cobalt powder is obtained by electrolysis of an aqueous solution of cobalt salts (mainly Sulfate cobalt) using an electrode consisting mainly of platinum or pure cobalt, and combining the two types of anodes forming a specific platinum section that does not produce an electrolytic screen over cobalt located at that location.
After the prototype of the simultaneous application of soluble and insoluble anodes reduces the intensity of the dissolution of cobalt plates by reducing their working area. At the same time, the specific method for obtaining cobalt powder, there is a difficulty in choosing the optimum ratio of the square of the soluble anode to the surface of the insoluble anode (Sp: Sn), which makes it possible to keep the concentration of cobalt ions constant during the electrolysis process , In addition, the cobalt plate dissolves over time, thus altering its working surface, which in turn leads to a disturbance of the area ratio of the anodes of the two species and a change in the content of dissolved metal cations.
The aim of the proposed technical solution is the alignment of the cathode and the anode (soluble anode) o of the Dov-current in the absorption of metal powder by electrolysis and thus the exclusion of a time-consuming adaptation of the electrolyte in the industrial application of this method.
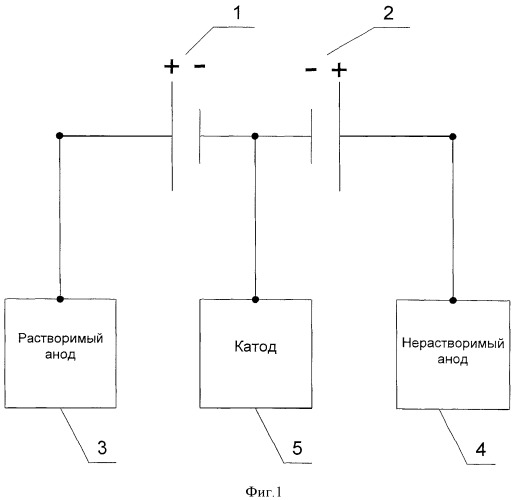
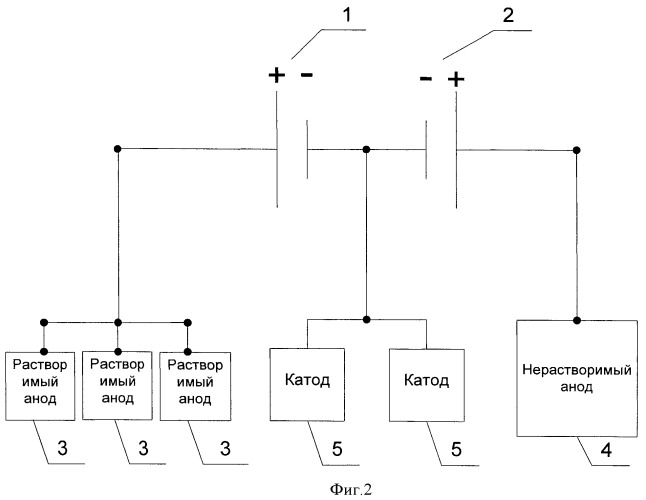
This problem is solved in that in the known method, including electrocoating powder metal aqueous solution of the salt with the use of soluble and insoluble anodes at the same time, encourages them to be connected to different sources of power supply. The claimed technical solution is illustrated by the diagram shown in Figures 1 and 2. Figure 1 shows a variant of the electrical supply circuit of a cathode, one of the soluble anode and an insoluble anode, 1 being the first current source 2 to the second current source, 3 - soluble anode, 4 - insoluble anode 5 being the cathode. Figure 2 shows a variant of the electrical supply circuit of the two cathodes, three soluble anodes and one insoluble anode, where 1 is the first current source 2 to the second current source, 3 - soluble anode, 4 - insoluble anode 5 is the cathode.
According to the claimed technical solution, by setting the ratio of current densities (current) soluble (3) and insoluble (4), such a power supply circuit enables the anode at a constant cathodic current density or current at the cathode (5) the electrolysis process, the equality of the anode current output ( soluble anode) and the cathode current output, that is a constant concentration of metal ions in solution. The constancy of the cathode PhotoStitch is achieved by the constancy of the cathode area and the sum of the forces of the currents supplied by the current source (1) and (2):
IIT + IT2 = total,
wherein Itotal- the total current in the general area of the cathode circuit, and;
IIT-current-soluble anode supplied by the source (1), and;
IT2 electroless insoluble anode supplied by a source (2), A.
The value of Itotal defines a constant cathodic current density at a constant cathode area. For example, if the electrolysis process is to increase the concentration of metal cations, it is necessary to reduce the dissolution intensity of the soluble anode by reducing the current, eg, 10 and the current source (1), and increasing the current or current source (2) 10 A, is reached. The sum of the forces of the power source (1) source (2) remains constant.
In the application of the proposed technical solution for the installation of the electrolysis can be any suitable combination and the number of cathodes, soluble and insoluble anodes. The claimed technical solution can be applied to obtain any metal powder by electrolysis of an aqueous salt solution of the corresponding metal. The proposed solution is tested after obtaining the electrolytic powders of nickel, cobalt and copper.
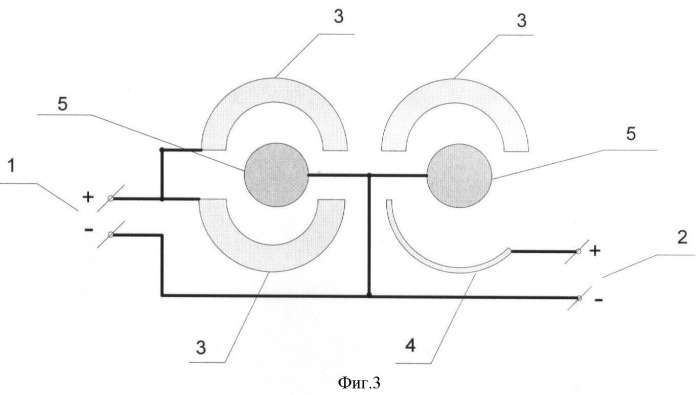
An example of a specific application of ingenuity those who find practical solutions can serve as a description of the technological process of making cobalt powder in a conical mechanized bath with two rotating cylindrical cathodes. As anodes simultaneously with soluble anodes charging type (titanium basket and cobalt metal) and the insoluble anode oxide-ruthenium-titanium anode), which are connected to different power sources. Power supply circuit cell according to FIG. 3, where 1 is the first current source 2 to the second current source, 3 - soluble anode, 4 - insoluble anode, 5 is cathode. The electrolysis takes place in an electrolyte that contains cobalt sulphate in the amount of 35 g / l and ammonium sulphate as a buffer additive in an amount of 50 g / l when the pH of the electrolyte is 5,8 ± 0,6 and a temperature of 50 Was ± 5 ° C. The cathodic current density is equal to 34 A / DM2. When you apply an electrical voltage to the electrodes, perform the following electrochemical reaction:
at the cathode:
With2 ++ 2E → Co;
2N ++ 2E → H2 ↑;
on the soluble anode:
Co-2E → Co2 +;
H2O- 2E → 2H ++ 0.5o2 ↑;
on the insoluble anode:
H2O - 2E → 2H ++ 0.5o2 ↑.
Cobalt in powder form on the cathode is removed every 30 minutes with a scraper. Scraper, scraping powder immediately with both electrodes, after a certain time brought into contact with the surfaces of the cathodes, without switching off the load. Cobalt powder, which has accumulated on the bottom of the bath for 24 hours, filtered from an electrolytic suction filter, washed with distilled water, dried, thermoablatively in a stream of hydrogen and sieved. The average particle size of the obtained cobalt powder is equal to 20 μm. Figure 4 shows a photograph of cobalt powder prepared by the above method, which confirms its uniformity in the application of the proposed technical solution.
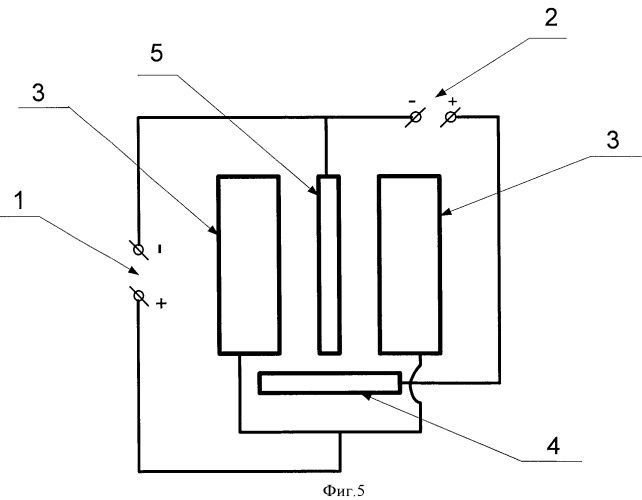
A second example of a specific application of the proposed technical solution can serve as a description of the technological process of manufacturing nickel powder in a conical tub with a titanium cathode in the form of a plate. As anodes simultaneously with soluble anodes charging type (titanium basket and nickel metal and insoluble anode oxide-ruthenium-titanium anode), which are connected to different power sources. Power supply circuit cell according to FIG. 5, wherein 1 is the first current source 2 to the second current source, 3 - soluble anode, 4 - insoluble anode, 5 cathode in the form of a plate. The electrolysis is carried out in an electrolyte which contains nickel sulphate in the amount of 15 g / l and a buffer additive, the ammonium sulphate in an amount of 65 g / l, at a pH of the electrolyte of 5,5 ± 0,5 and at a temperature of 50 ± 5 ° C. Cathodic current density of 22 A / DM2. When applying an electric current to the electrodes, according to electrochemistry, the Kie reaction occurs:
at the cathode:
Ni2 ++ 2E → Ni;
2H ++ 2e → H2 ↑;
on the soluble anode:
Ni - 2E → Ni2 +;
H2O- 2E → 2H ++ 0.5o2 ↑;
on the insoluble anode:
H2O - 2E → 2H ++ 0.5o2 ↑.
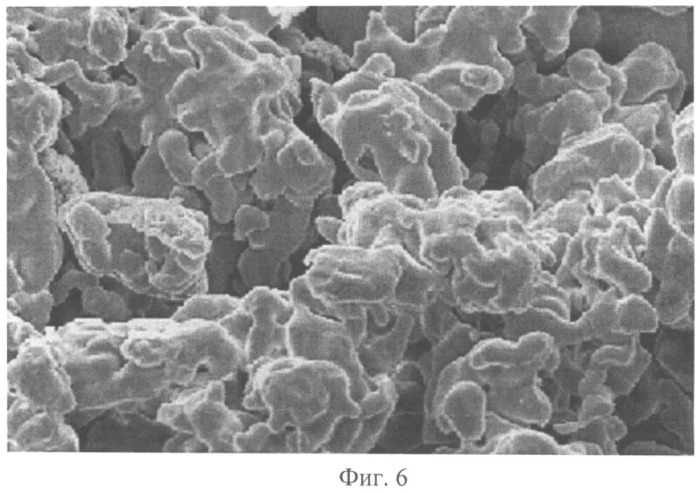
Nickel formed in powder form on the cathode is removed every 60 minutes with an automated mechanical stripping device of the “guillotine” type. Scraper, scraper powder immediately on both sides of the electrode, was set in motion after a certain time to relieve the electrodes. Nickel powder that has accumulated on the bottom of the bath over 24 hours, filtered from the electrolyte on a suction filter, washed with distilled water, dried, thermo-ablated in a stream of hydrogen and sieved. The average particle size of the nickel powder obtained corresponds to 25 μm. Figure 6 shows a photograph of the nickel powder obtained by the above process, which confirms its uniformity when the proposed technical solution is applied.
A third example of a specific application of the proposed technical solution can serve as a description of the technological process for the extraction of copper powder in a conical tank with a titanium cathode in the form of a rod. The insoluble anodes made of cast copper and the lead anode, which are connected to different power sources, are used as anodes. Power supply circuit cell according to Fig. 7, wherein 1 is the first power source 2 to the second power source, 3 - soluble anode, 4 - insoluble anode, 5 cathode in the form of a rod. The electrolysis takes place in an electrolyte which contains copper sulphate (20 g / l and added buffer), which is absorbed in sulfuric acid in an amount of 50 g / l at an electrolyte temperature of 5 ± 150 ° C. The cathodic current density is equal to 32 A / DM2. When you apply an electric current to the electrodes, you will perform the following electrochemical reaction:
at the cathode:
Cu2 ++ 2E → Cu;
2N ++ 2E → H2 ↑;
on the soluble anode:
Cu - 2E → Cu2 +;
H2O- 2E → 2H ++ 0.5o2 ↑;
on the insoluble anode:
H2O - 2E → 2H ++ 0.5o2 ↑.
The copper produced at the cathode in powder form is removed by hand every 60 minutes with a special wiper with idle to the electrodes. Copper powder, which has accumulated at the bottom of the bath within 24 hours, filtered on a suction filter from the electrolyte, washed with distilled water, dried, thermoablatively dried in a stream of hydrogen and sieved. The average particle size of the obtained copper powder is equal to 15 μm. On Fig presents a photo of the copper powder, which by the above method, colorattribute its uniformity in the application of the proposed technical solution.
The proposed solution allows alignment of the cathode and anode (soluble anode) output current in the uptake of metal powder by electrolysis, thereby reducing the time consuming operation of the electrolyte in the industrial application of this process.
A method for obtaining electrolytic powders of metals by electrolysis of an aqueous solution containing a salt of the corresponding metal and buffer additives, with simultaneous use of soluble and insoluble anodes, characterized in that the soluble and insoluble anodes are connected to a separate power source In order to control the dissolution of the soluble anode during the electrolysis process and the concentration of metal ions in solution by adjusting the ratio of the anode, force current-soluble and insoluble anodes to a constant value of cathodic current density, which determines the consistency of the cathode area and the amount of forces of the cathode Reached current in the soluble and insoluble anode.
ISE / Arndt Uhlendorff - October 2019